CHAPTER 21 Female Reproductive System
CLINICAL CONSIDERATIONS
There are a wide variety of indications for imaging the female pelvis. From acute pelvic pain and abnormal uterine bleeding (AUB) to identifying and characterizing pelvic masses or potential causes of infertility, imaging plays a crucial role in diagnosis and management. However, imaging studies obtained to evaluate pelvic pathology can often be difficult to interpret in isolation. The pelvis contains elements of the gastrointestinal and urinary tracts, as well as the reproductive system, all of which can be affected by congenital, inflammatory, and neoplastic processes. The dissemination of pelvic inflammation or neoplasm via the peritoneal cavity or extraperitoneal spaces can make the organ of origin difficult or impossible to determine with imaging alone. Furthermore, the pelvic structures and organs lie in close proximity to one another, and intervening fat planes can be subtle or absent on imaging studies, particularly in thin patients. This explains the difficulty often encountered in distinguishing between pelvic inflammatory disease and appendicitis. Therefore, radiologists must rely on accurate clinical information to improve specificity and ensure appropriate management.
Certain clinical data should be sought by the radiologist when interpreting imaging studies of the female pelvis. Appropriate questions to ask are included in Table 21-1.
Table 21-1 Appropriate Inquiries When Interpreting Female Pelvic Imaging Studies
| Inquiry | Significance |
|---|---|
| Clinical History | |
Ovarian cyst rupture/hemorrhage and torsion of ovary or fibroid often present with sudden onset of symptoms | |
| Physical Findings | |
| Laboratory Data | |
| CA-125 | |
β-hCG, β-Human chorionic gonadotropin; PID, pelvic inflammatory disease.
The differential diagnosis of acute pelvic pain is discussed in Chapter 8. Therefore, after a brief discussion of female pelvic anatomy, this chapter focuses primarily on nonacute abnormalities of the female pelvis.
Anatomy of the Female Reproductive System
Uterus
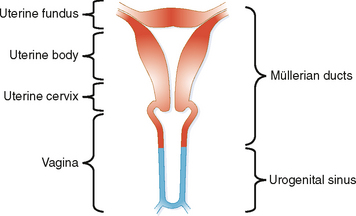
The uterus is a muscular pouch that forms from paired müllerian ducts. The ducts first fuse; then some of the intervening tissue resorbs to form the fallopian tubes, uterus, and upper one-third of the vagina (Fig. 21-1). Most congenital anomalies of the uterus and vagina result from either failure of the ducts to fuse or failure of the intervening tissue to resorb. The mucosal lining of the uterus is called the endometrium. The muscular wall is the myometrium. The uterine fundus protrudes into the peritoneal space, encased by a blanket of peritoneum that forms the broad ligament on either side.
Fallopian Tubes
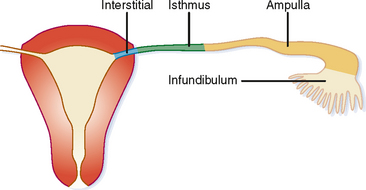
The fallopian tubes are a continuation of the müllerian ducts that form the uterus and provide communication between the peritoneal cavity and the outside world. It is necessary, of course, for the two worlds to meet for fertilization to occur. The fallopian tube averages 10 cm in length and can be subdivided into four segments (Fig. 21-2).
Pelvic Floor
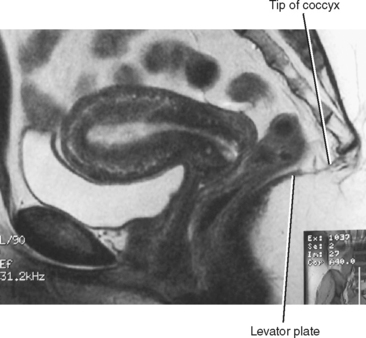
The bony pelvis is basically shaped like a large ring. The pelvic organs are supported by a complex set of fascial, ligamentous, and muscular structures, which are attached to each other and to the bony framework of the pelvis, to counter the effects of gravity (and Valsalva). The levator ani complex is a key element of this slinglike support system and has multiple components, including the puborectalis and iliococcygeus muscles. The puborectalis provides anterior support, attached to either side of the pubic bone near the symphysis, and makes a sling around the anus, keeping the anus, vagina, and urethra bound to the pubis (Fig. 21-3). The iliococcygeus provides posterior and lateral support through multiple interdependent osseous and fascial attachments. The levator plate is a posterior midline condensation of the ileococcygeus best seen as a linear attachment to the coccyx on sagittal magnetic resonance (MR) images (Fig. 21-4).
Perineum
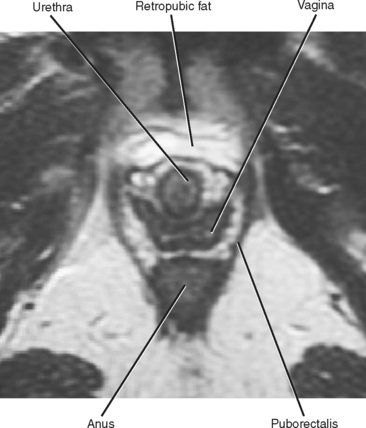
The urethra, vagina, and rectum pass through the U-shaped levator hiatus. The urethra is most anterior and often has a target-like appearance on T2-weighted MR images. Just posterior to the urethra is the vagina, which typically has an H or butterfly shape. The anus is posterior to the vagina. The urethra and vagina end in the external urethral meatus and vaginal introitus, respectively, both of which lie in the vestibule between the labia minora. Skene’s glands adjacent to the urethra produce mucus that lubricates the mucosal surface. Embedded within the posterior aspect of the labia minora are the paired Bartholin glands, with ducts that drain on either side of the vaginal introitus.
NORMAL IMAGING APPEARANCE OF THE FEMALE PELVIS
Ultrasound
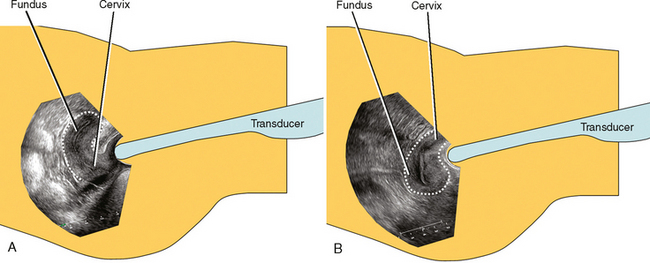
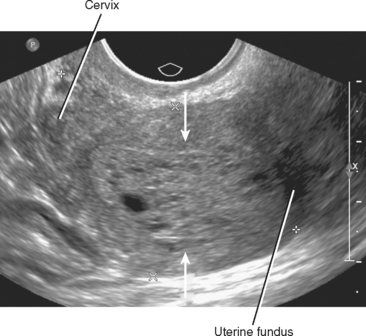
Ultrasound (US) is the first-line modality for most applications in the female pelvis. Transvaginal ultrasound (TVUS) provides high-resolution images of the uterus. With the transducer only millimeters away, US provides excellent detail of the endometrium and myometrium in most patients. The orientation of TVUS images can be disorienting. To the uninitiated, it sometimes helps to rotate images of the uterus clockwise 90 degrees (Fig. 21-5).
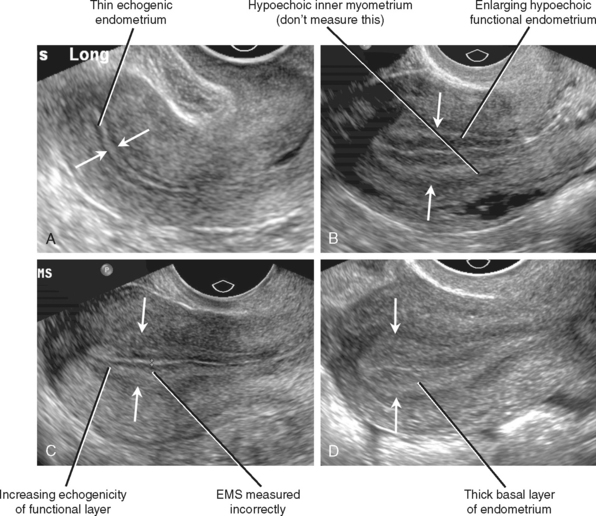
Hormonal influences on the endometrium result in a variable appearance of the endometrial stripe (EMS) on TVUS during the alternating proliferative and secretory phases of each menstrual cycle (Fig. 21-6). A hypoechoic layer of functional myometrium is usually seen surrounding the echogenic apposed endometrial mucosa; it is important that this dark line be omitted from the EMS measurement. During the proliferative phase, the hypoechoic line of the inner myometrium is outside all three echogenic endometrial lines. In premenopausal women, the EMS typically measures up to 8 mm during the proliferative phase and up to 14 mm during the secretory phase. After menses, the EMS becomes thin (2-3 mm). Because of variability, absolute EMS thickness is less important than uniformity in premenopausal women.
The postmenopausal endometrium becomes atrophic, usually measuring 2 to 3 mm in thickness with uniformly increased echogenicity (Fig. 21-7). A cutoff for EMS measurement of 5 mm or less is used to effectively exclude malignancy with a sensitivity of 96% (this is discussed in greater detail later in the Imaging Evaluation for Abnormal Uterine Bleeding section). Little data have been reported regarding the significance of an EMS measurement of greater than 5 mm found incidentally in an asymptomatic postmenopausal woman.
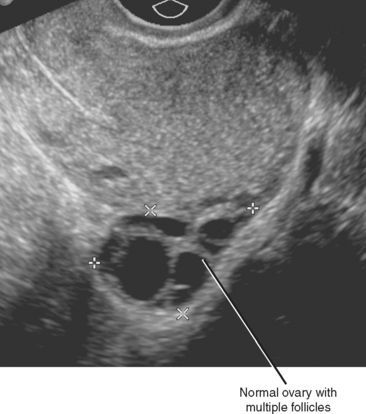
Despite variable location of the ovaries, they can be identified with transvaginal sonography in nearly all premenopausal women. The ovaries can be more challenging to identify after menopause but are still identified in the majority of women. The normal ovary contains multiple small anechoic follicles, typically ranging from 1.0 to 2.5 cm (Fig. 21-8). Most consider a cystic structure in the ovary that exceeds 2.5 cm in diameter to be a cyst, and most of these are functional cysts that will resolve over time. Depending on the phase of the ovulatory cycle, a corpus luteum may be present. With US, the corpus luteum typically has an irregular, thick wall that demonstrates increased flow on color Doppler. This appearance has prompted use of the term ring of fire.
Computed Tomography
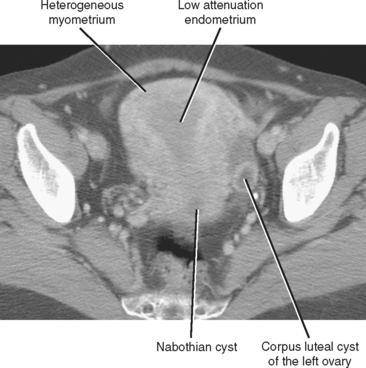
More rapid scan acquisition with MDCT often results in an earlier phase of uterine enhancement compared with single-detector CT. Although the impact of hormonal cyclic changes on the US appearance is wellrecognized, few consider that the vascular changes that contribute to transitions from proliferative to secretory phases result in different patterns of uterine enhancement on CT. Heterogeneous uterine enhancement on MDCT is usually a normal finding (Fig. 21-9). The normal endometrium appears hypoattenuating relative to the myometrium on unenhanced CT images, and this relation persists through the portal phase of imaging after administration of intravenous contrast material. This appearance is often confused with endometrial fluid by inexperienced CT readers.
The ovaries can be found by CT in virtually all cases. On axial sections, note the region where the uterus changes from oval shaped to tapered as the contour becomes defined by the broad ligament. In most cases, this tapering causes the uterus to “point” toward each ovary, or at least to a thin band of broad ligament that can be followed to the ovary. The ovarian veins are readily identified in most cases, and following each ovarian vein inferiorly should demonstrate where it joins the suspensory ligament of the ovary, usually between the ovary and iliac vessels. CT reveals less detail regarding the normal ovary compared with US. Often, individual follicles cannot be discerned, or only the dominant follicle is seen by CT.
Magnetic Resonance Imaging
MRI is used to image the female pelvis when US and CT are inconclusive. MR is also the most accurate imaging modality for characterizing congenital uterine anomalies and for staging most gynecologic malignancies. Cinematic MRI can be useful for the evaluation of pelvic floor laxity. When performing MRI of the pelvis, it can be helpful to have the patient fast or to administer glucagon to reduce artifact from bowel peristalsis. For evaluation of the uterus, imaging planes should be chosen relative to the uterine axes rather than relative to the pelvis (Fig. 21-10).
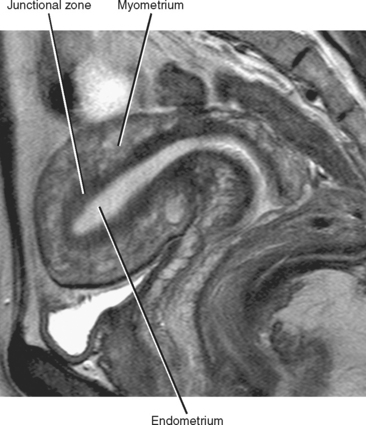
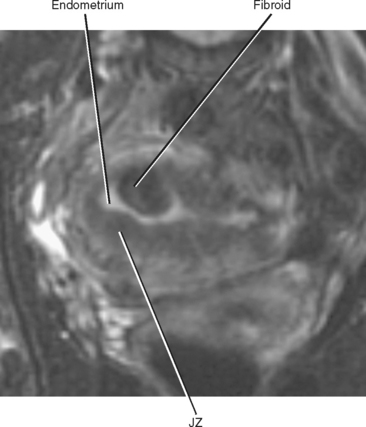
On T2-weighted MR images, the uterus can be divided into three distinct layers: the central high-signal-intensity endometrium, the low-signal-intensity junctional zone, and the outer intermediate-signal-intensity myometrium (Fig. 21-11). Thickness of the endometrium varies throughout the menstrual cycle in premenopausal women, with a width of up to 14 mm considered normal during the secretory stage of the menstrual cycle. In postmenopausal women, an endometrial thickness of 5 mm or less is considered normal.
The junctional zone, regarded as a distinct stratum by MRI, is actually the basal layer of the myometrium. This layer is easy to identify on T2-weighted images because it appears as a smooth, dark band between the hyperintense endometrium and the intermediate signal myometrium. The junctional zone should appear smooth, and a thickness of less than 8 mm is considered normal. Zonal anatomy often becomes less distinct in postmenopausal women (Fig. 21-12).
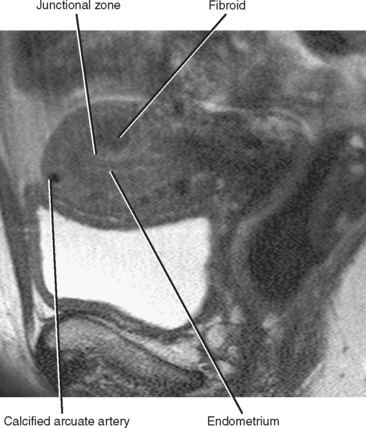
Figure 21-12 Sagittal T2-weighted magnetic resonance image of a postmenopausal uterus. Zonal anatomy is much less distinct than in Figure 21-11.
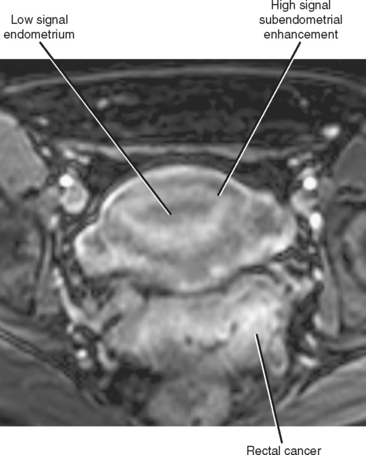
Uterine zonal anatomy cannot be distinguished on unenhanced T1-weighted images. After contrast administration, the myometrium enhances briskly, whereas the endometrium undergoes more delayed progressive contrast enhancement, eventually becoming isointense or hyperintense to myometrium. In postmenopausal women, a zone of normal subendometrial enhancement on postcontrast T1-weighted images is often more helpful in demarcating the transition from endometrium to myometrium than is the often inapparent junctional zone on T2-weighted images (Fig. 21-13).
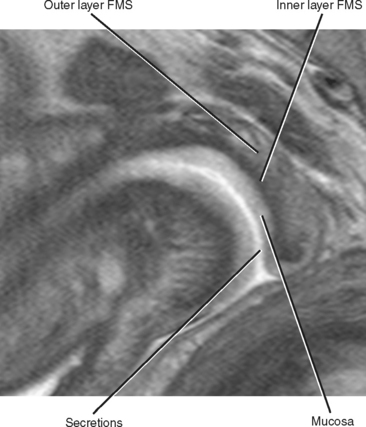
The zonal anatomy of the cervix is best appreciated on T2-weighted imaging sequences. From central to peripheral, the three anatomic zones include the endocervix, the inner fibromuscular stroma (FMS), and the outer FMS (Fig. 21-14). The endocervical mucosa, secretions, and plicae palmatae (mucosal folds) appear as increased signal intensity centrally within the cervix on T2-weighted images. The inner FMS has low signal intensity, likely the result of a densely packed zone of fibroblasts and smooth muscle cells. The outer FMS, composed of more loosely packed tissue, is low-to-intermediate signal intensity on T2-weighted images.
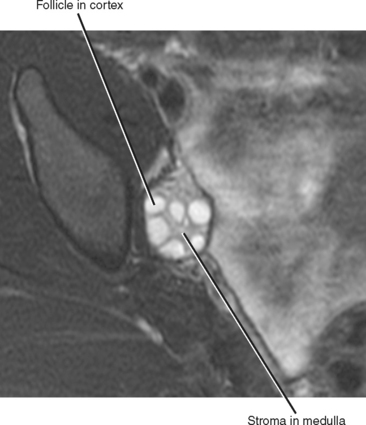
The ovary also has an identifiable zonal anatomy on T2-weighted imaging with a low-signal cortex peripherally, an intermediate-signal medulla centrally, and high signal intensity cysts and follicles (Fig. 21-15). In normal premenopausal women, the appearance of the ovaries varies with the menstrual cycle. Normal follicles range in size from several millimeters up to 2.5 cm, and should appear of homogenously low signal intensity on T1-weighted images and high signal intensity on T2-weighted images. Dominant follicles become corpus lutea in the secretory phase of the menstrual cycle, often displaying smooth, thick walls that enhance avidly after intravenous contrast administration. The corpus luteum involutes if pregnancy does not occur, and imaging at this stage reveals a convoluted cyst contour. In postmenopausal women, the ovaries progressively decrease in size over time and appear as oval structures of low signal intensity. Dormant follicular cysts can be seen in normal postmenopausal ovaries as small, simple cysts.
Hysterosalpingography
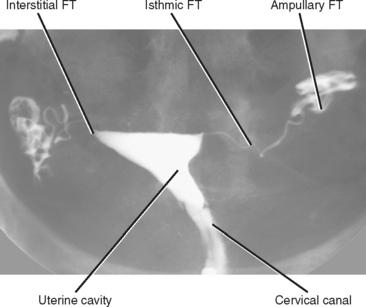
Injection into the cervix opacifies a funnel-shaped endometrial canal that should have a smooth appearance without filling defects (Fig. 21-16). The fallopian tubes opacify next and should be thin and gracile proximally, then gradually widening until contrast spills from the ampullary portion into the peritoneal space.
IMAGING EVALUATION FOR ABNORMAL UTERINE BLEEDING
Although TVUS is sensitive for detecting endometrial abnormalities, it is often unable to definitively distinguish between benign and malignant processes. Cases of abnormally thickened endometrium should, therefore, be investigated further with sonohysterography, endometrial biopsy, or hysteroscopy. Hysteroscopy is considered by many to be the gold standard for endometrial evaluation, but it is costly and invasive. Current techniques of pipette endometrial biopsy can be performed during an office visit but provide only a small, blind sample of endometrium. This is effective for diffuse abnormalities of the endometrium but is less sensitive for focal abnormalities.
Saline infusion sonohysterography (TVUS with saline infusion) is a minimally invasive procedure that provides a more detailed evaluation of the endometrium. Infusion of saline allows evaluation of the endometrium as a single layer, rather than the opposed layers that comprise the usual EMS. This improves differentiation between focal and diffuse disease, and improves characterization of subendometrial lesions (Fig. 21-17). For the procedure, a speculum is used to visualize the cervical os, which is then cannulated with a small catheter. Catheters in use range from simple pediatric feeding tubes to HSG catheters with retention balloons. The speculum is then withdrawn, and the endovaginal US probe is inserted parallel to the catheter. The endometrial cavity is then filled with sterile saline (usually 5-10 mL). In a study comparing sonohysterographic findings with subsequent hysteroscopy and pathology, the imaging results were found to be congruent with the pathology results in 92.6% of the 122 patients studied.
CHARACTERIZING UTERINE MASSES
Endometrial Masses
Types of Endometrial Masses
ENDOMETRIAL HYPERPLASIA
Endometrial hyperplasia is excessive proliferation of the endometrial glands, focal or diffuse, and is thought to result from unopposed estrogen stimulation. Such stimulation is commonly seen in the setting of tamoxifen therapy in patients with breast carcinoma. Cellular atypia can be present, in which case the risk for development of endometrial carcinoma is increased. Hyperplasia usually appears as enlargement of the EMS on TVUS or MRI, a nonspecific finding. In some cases, cystic dilatation of the endometrial glands results in anechoic spaces on TVUS (Fig. 21-18). Cystic endometrial hyperplasia is particularly common with tamoxifen use. On MRI, these cystic spaces appear as small foci of increased signal intensity on T2-weighted images, which do not enhance after contrast administration.
ENDOMETRIAL POLYPS
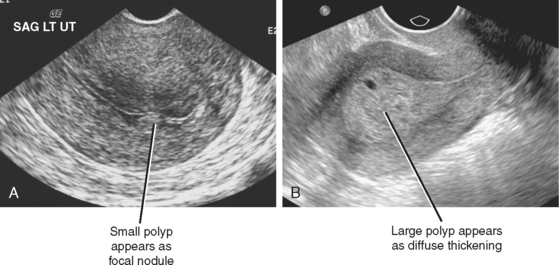
Endometrial polyps can be considered as a more focal form of endometrial hyperplasia. Although they can be multifocal, most are sessile or pedunculated solitary lesions. Polyps typically appear as diffuse or focal echogenic thickening of the endometrium on TVUS, and most occur in the uterine fundus and cornua (Fig. 21-19). Polyps tend to be nearly isoechoic to endometrium and can have a frondlike appearance on sonohysterography. When fluid is present in the endometrial canal, the margins of the polyp might be defined and a stalk identified in some cases.
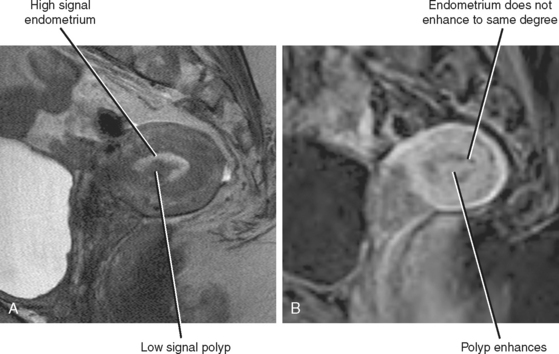
The MR signal characteristics of polyps are variable. Polyps are often hypointense relative to the adjacent endometrium on T2-weighted images but can be isointense (Fig. 21-20). Both solid and cystic components are often visible. Polyps demonstrate variable enhancement on postcontrast images, often enhancing more than adjacent endometrium on early dynamic images but less than the endometrium on delayed images. Although only occasionally visible on MRI, identification of a stalk strongly suggests the diagnosis. Whereas subtle discriminators may allow differentiation of polyps from endometrial cancer in some cases (fibrous core, intratumoral cysts, endometrial cysts, and lack of muscular invasion), overlap is present in the appearances of benign polyps and early endometrial carcinoma.
SUBMUCOSAL FIBROIDS
Submucosal fibroids are a common cause of AUB. In some cases, a large fibroid is predominantly myometrial, with only a small submucosal component. In others, a pedunculated submucosal fibroid mimics the appearance of an endometrial polyp. In such cases, US or MR aids in the diagnosis.
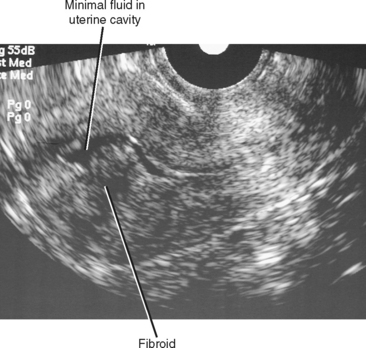
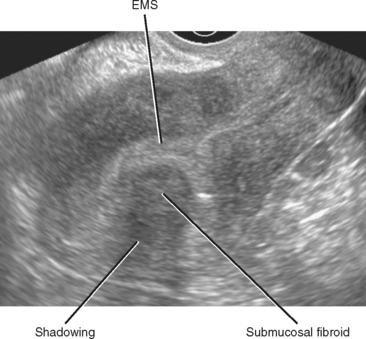
On TVUS, submucosal fibroids usually appear as a spherical hypoechoic mass that distorts the EMS (Fig. 21-21). Acoustic shadowing is common but not always present. MRI demonstrates similar distortion of the endometrial canal (Fig. 21-22). Fibroids usually have decreased signal intensity compared with polyps on T2-weighted images.
Staging Endometrial Carcinoma
Endometrial carcinoma staging is performed according to surgical and pathologic criteria defined by the International Federation of Gynecology and Obstetrics (FIGO). Corresponding TNM staging corresponds to FIGO criteria. Tables 10-8 and 10-9 outline both staging systems. In most centers, hysterectomy is performed in all cases of endometrial carcinoma, unless there is evidence of advanced metastatic disease. Pretreatment imaging has played little role. Recently, considerable literature has demonstrated accuracy of pretreatment imaging with MRI, and this has become more common in large cancer centers.
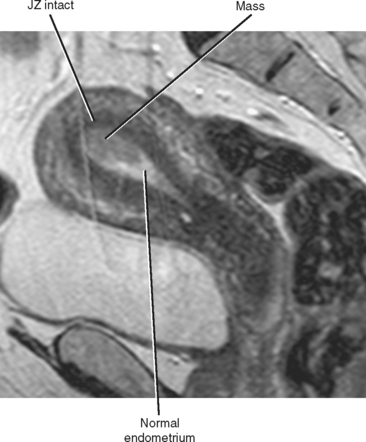
At many centers in the United States, MRI is the preferred imaging modality for preoperative staging of endometrial carcinoma. Stage IA disease (confined to the endometrium) can be suggested when an endometrial mass fails to extend into the junctional zone on T2-weighted images (Fig. 21-23) or the zone of subendometrial enhancement on dynamic contrast-enhanced T1-weighted images (helpful in postmenopausal women with poor zonal anatomy on T2-weighted images).
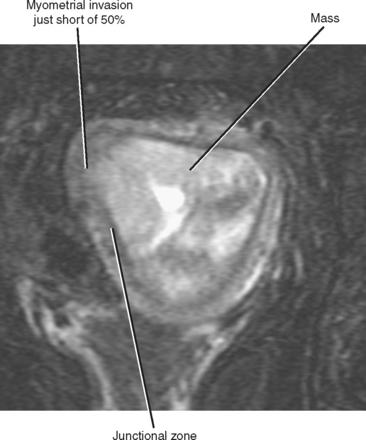
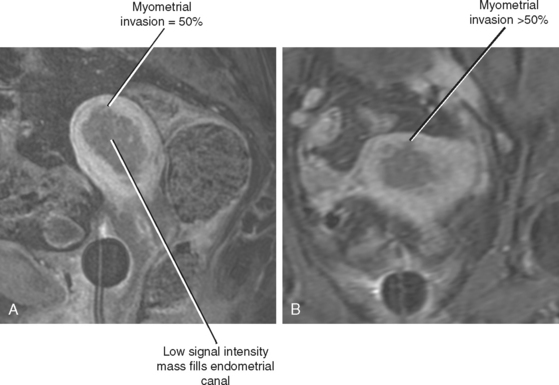
Interruption of the normally dark continuous junctional zone by higher signal intensity tumor is considered a sign of myometrial invasion (Fig. 21-24). Determining the depth of myometrial invasion (greater or less than 50%) is best achieved with images obtained axial and/or sagittal with respect to the uterus (Fig. 21-25). T1-weighted imaging after administration of gadolinium chelates is complementary and perhaps most useful when the junctional zone is indistinct. Endometrial carcinoma typically enhances in a delayed manner relative to myometrium (similar to normal endometrial enhancement). Therefore, invasive endometrial carcinoma will appear hypointense relative to the enhancing myometrium (see Fig. 21-25). Factors that may confound evaluation of myometrial invasion by MRI include an abnormal junctional zone caused by adenomyosis, distortion of the endometrial canal and myometrium because of fibroids, and thinning of the uterine wall because of large tumors.
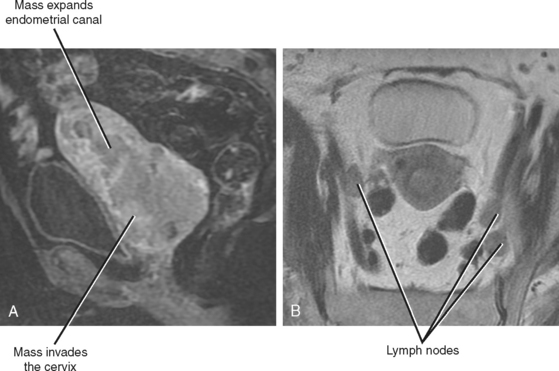
When tumor extends into the cervix, stage II disease is diagnosed. Extension beyond the uterus denotes stage III disease if contained in the true pelvis (Fig. 21-26), and stage IV disease if extending beyond the true pelvis or invading the bladder or rectum.
Myometrial Masses
Fibroids
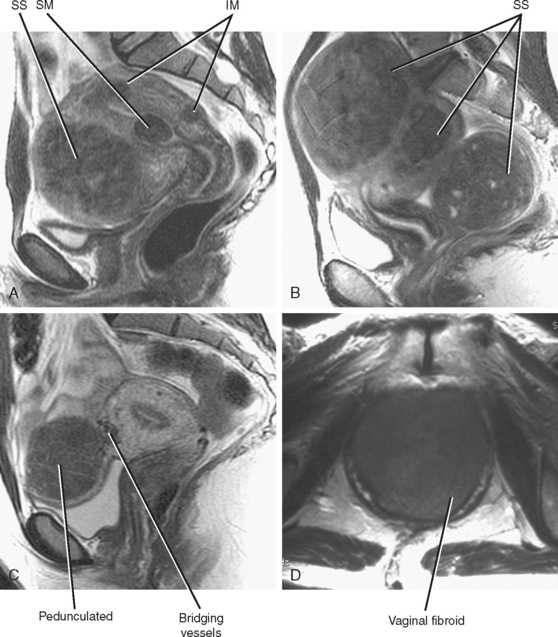
The most common lesion found within the uterus is the leiomyoma, or fibroid, which is a benign neoplasm composed of smooth muscle cells. Fibroids (easier than saying “leiomyomata”) occur in greater than 25% of women older than 35 years and are classified according to their location (Fig. 21-27). Classification is helpful in determining whether a given fibroid is likely to account for the patient’s symptoms and what type of therapy (if any) may be appropriate.
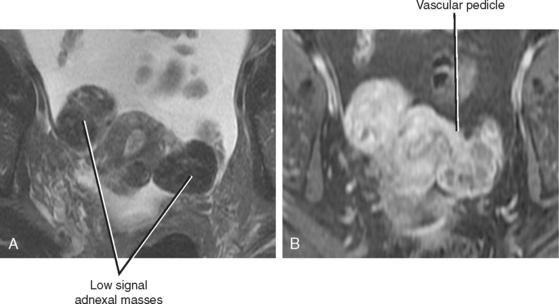
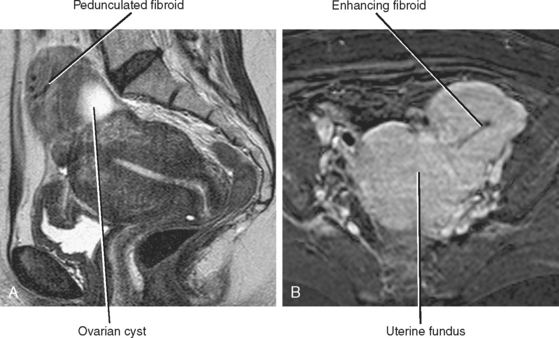
When a fibroid displaces the uterine serosa outward, it is considered to be subserosal. The appearance of subserosal fibroids ranges from a bulge in the uterine contour to an entirely pedunculated mass that can mimic an adnexal tumor (Fig. 21-28). Fibroids can also be found within the broad ligament, appearing completely detached from the uterus. Visualizing the connection of the mass to the uterus helps differentiate a pedunculated subserosal fibroid from an ovarian mass. Fibroids arise from the uterus and, therefore, are typically supplied by branches of the uterine artery. The bridging vascular sign refers to visualization of a vascular pedicle extending from the uterus to the fibroid on US or MR images. Ovarian tumors are typically supplied and drained by the ovarian vessels, although ovarian artery supply is occasionally seen with fibroids.
Fibroids vary in sonographic appearance. Most fibroids are heterogenous but predominately hypoechoic in appearance. Acoustic shadowing is common. Often, a discrete mass is difficult to define, and distortion of the uterine contour with posterior shadowing is the clue to the presence of fibroids. Small, diffuse tumors can cause the uterus to appear enlarged and heterogeneous. When present, calcifications can be seen as echogenic foci or a hyperechoic peripheral rim. Cystic spaces can be seen with necrosis.
With MRI, smaller solitary fibroids are usually well-defined and demonstrate characteristically low signal on T2-weighted images compared with the myometrium. A peripheral halo of increased T2 signal has also been described and attributed to perilesional edema, dilated veins, or dilated lymphatics. Fibroids are often cellular, enhancing as much as the myometrium on CT or MRI (Fig. 21-29).
As fibroids grow larger than 3 to 5 cm, they commonly undergo degeneration
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree