KEY POINTS
Data collected from Doppler velocity recordings using both spectral and color Doppler mode confirm the fundamental aspects of the fetoplacental circulation. Changes in placental vascular resistance, cardiac contractibility, vessel compliance, and blood viscosity alter the normal dynamics of fetal cerebral circulation.
Reference values have been established for the main cerebral vessels. During the last trimester of normal pregnancies, the values of Doppler waveform indices decrease in all main cerebral vessels. After birth, vascular resistance decreases, and later stabilizes. Cerebral autoregulation persists from fetal to postnatal life, with low waveform indices in cerebral vessels of growth-retarded fetuses and neonates. The low indices indicate decreased cerebrovascular resistance, representing the redistribution of flow, or the brain-sparing effect.
Combined parameters recorded from different vascular beds may provide support for the diagnosis of significant hemodynamic changes and are of prognostic value in predicting fetal outcome such as fetal IUFGR and Rh disease.
As far as imaging of the vascularization (arterial and venous) of the prenatal brain, it should be clear that no brain scan should be considered complete without looking at its main vessels or at least at the pericallosal artery on a median section. Should such a protocol be too complicated to achieve in all anomaly scans, it is important to scrutinize brain vascularization in each and every case of suspected brain anomaly.
Three-dimensional power Doppler US technology, especially combined with high-frequency TVS, as described in this and other chapters, is the most preferable noninvasive and reproducible imaging method to be used. The accurate information about deviant brain vascularity may help to render proper obstetric, neurologic, and neurosurgical management.
Recent sonographic advances have contributed to accurate and reliable visualization of intrauterine vascularity. The first breakthrough in the assessment of fetal circulation was the Doppler system. Technical developments in Doppler ultrasound (US) equipment, especially highly sensitive color Doppler, power Doppler, and bidirectional power Doppler imaging techniques, have made it possible to study the fetal circulatory system, including cerebral vascularization. Early fetal circulation has been demonstrated by conventional two-dimensional (2D) color Doppler since the 1990s.1 Fine vessels inside the choroid plexus were depicted and assessed in 1994.2,3 Two-dimensional color/power Doppler combined with transvaginal sonography (TVS) became a powerful tool to demonstrate early fetal vascularization.4,5 Embryonal/fetal development is amazingly rapid, and the structure of the brain changes throughout pregnancy. Introduction of three-dimensional (3D) power Doppler technology in the late 1990s enabled visualization of intracranial vessels. This assessment, combined with TVS, has provided 3D sonoangiographic images, and adds useful information in the prenatal evaluation of normal brain development, vascular malformation, and tumoral vascularity.6,7
Vascular endothelial cells cover the entire inner surface of blood vessels and are influenced by vascular endothelial growth factor (VEGF), which is a potent angiogenic factor working as an endothelial cell-specific mitogen and exerting a trophic effect on neurons and glial cells. Both of these activities are essential during central nervous system (CNS) vascularization, development, and repair. In these cells, VEGF expression developmentally regulates and is correlated with angiogenesis, which in turn responds to the high metabolic demands of the developing fetal brain.8
From the beginning of cardiac development, the cranial parts of the truncus arteriosus enlarge and transform into the aortic sac, which later gives rise to the aortic branches. After splitting of the truncus arteriosus by the aorticopulmonal septum, the primoridal aortic sac becomes part of the ventral aorta, while a pair of the dorsal aortae develop independently of the cardiac tube. During later development, extensive changes occur, leading to loss of the symmetrical state of these primitive vessels. In brief, the common aortic artery, along with the first part of the internal carotid artery (ICA), arises from the third aortic branch, while the rest of the vessels develop from the cranial part of the dorsal aorta. The external carotid artery develops as a cranial part of the third aortic branch, while the vertebral arteries emerge from the dorsal aorta. In the development of cerebral fetal veins, the upper cardinal veins play the most important role, draining the blood from the cranial part of the embryo.
During fetal development (approximately 35 gestational days), the craniocerebral circulation is characterized by temporary connections between the primitive carotid and the paired dorsal longitudinal neural arteries (precursors of the vertebrobasilar system). They include persistent trigeminal, ottic, hypoglossal, and proatlantic intersegmental arteries. Normal embryonic development underwrites the regression of all these vessels totally. Sometimes the regression does not occur, resulting in a persistent trigeminal artery, which is the most frequent reason (85% of cases) of primitive carotid-to-basilar artery anastomoses.9
It should be said at the outset that the best way to study fetal circulation during its development is by employing 2D as well as 3D color and power Doppler technologies. Due to their physical limitations, transabdominal transducers cannot achieve high enough resolution to accurately display fine vessel anatomy. Therefore, high-frequency transvaginal probes have to be used for the best results.
Also the use of color and power Doppler should be used judiciously and sparingly during the first trimester according to the ALARA (as low as reasonably achievable) radiation safety suggestion (Title 10, Section 20.103 of the Code of Federal Regulators (USFNR).
Assessment of cerebral vascularization becomes possible from the seventh postmenstrual week of gestation, capturing low-velocity flow signals with an obvious absence of diastolic flow, from the periphery (walls) of the rhombencephalic cavity.3 At that stage the lateral ventricles are small evaginations located laterally and rostrally from the cavity of the diencephalon directly contiguous with the mesencephalon (Figure 15–1). The isthmus rhombencephali represents the connection to the rhombencephalic cavity and the future fourth ventricle. At this time of the evolution, the cavity of the rhombencephalon is the largest brain cavity visible in the embryonic head, as shown in Figure 15–1. From 8 postmenstrual weeks of gestation, it is possible to detect blood flow signals from the ICA and vertebral artery. At this stage, the rhombencephalic cavity (future fourth ventricle) decreases in size and moves toward the occipital part. Upon reaching the brain, the two ICAs turn dorsally and course alongside the diencephalon, giving off their branches. The ICA and its branches—the anterior cerebral artery (ACA), the middle cerebral artery (MCA), and the posterior communicating artery—form a polygonal-shaped communicating vessel called the circulus arteriosus, or the circle of Willis. The vessels arising from the circle of Willis supply the blood flow to the entire brain. Other vessels that form the circle are the vertebral arteries running between the transverse processes of C2 to C6 and giving a branch called the basilar artery and the posterior cerebral artery (PCA). The main components of the circulus arteriosus are present at Carnegie stage 16 (crown-rump [C-R] length of 10 mm, or 38 days postconception), and the circle is complete with Carnegie stage 16 (see Chapter 1). Arteries originating from the vertebral arteries are important for the supply of the cerebellum and brainstem. Within the symmetrical cerebellar hemispheres, it is possible to visualize the intracerebellar arteries. Blood flow signals from the intracerebellar arteries can be obtained from the ninth postmenstrual week of gestation, as shown in Figure 15–2. After the ninth postmenstrual week, the cerebellar superior, anteroinferior, and posterior arteries can be detected and separated from other cerebral arteries. These arteries are characterized by low to moderate impedance to blood flow. Three-dimensional power Doppler imaging presents the early vascular anatomy at the base of the skull with branches evolving laterally to the mesencephalon and cephalic flexure.10
With advancing gestational age, a progressive increase in blood flow velocity is noted for all cerebral arteries. Between the 9th and 10th postmenstrual weeks, the diastolic velocity component begins to emerge, but it is inconsistently present. From the 11th postmenstrual week, the end-diastolic blood-flow component of the velocity waveform begins to be consistently present. The posterior lateral choroidal artery derives from the PCA, whereas the lateral choroidal artery derives from the MCA and the ICA. Blood flow signals from the choroid plexus are obtained during the 9th and 10th postmenstrual weeks of gestation as subtle color and pulsed Doppler signals at the inner edge of the choroid plexus of the lateral ventricle. This developmental period is also the time of active neurogenesis. Choroid plexus vascularity during weeks 9 and 10 has two typical features: the presence of prominent venous blood flow signals and the absence of diastolic flow.2,11 In parallel, at this early age the cranial venous system, important for brain drainage, develops gradually. The venous circulation drains the blood into the dural sinuses, which meet in the confluence of the sinuses (torcular herophili) at the occipital pole of the skull, from where the blood flow drains into the jugular veins. As stated before, color/power Doppler and pulsed Doppler enable imaging and evaluation of the carotid artery8 and cerebral arterial blood flows. The MCA has been of particular interest to clinicians and clinical researchers alike and is therefore the objective of Doppler measurements and studies as the most important representative blood vessel of the brain. This interest is evident even at present, as measurements of blood flow profiles have stayed in daily clinical practice. The MCA is the largest branch of the ICA, supplying ~80% of the cerebral hemisphere. In middle and late pregnancy, the greater wings of the sphenoid bone, between the anterior and middle fossae, are good reference points for locating the MCA. It runs laterally in the sylvian fissure as a continuation of the intracranial carotid artery. This vessel consists of four segments—M1, M2, M3, and M4—and sends branches to the corpus striatum, the internal capsule, and the leuticulostriate nucleus. The preferable site for Doppler assessment is the M1 segment, as it maintains a more constant diameter. It then continues to cruise posteriorly over the surface of the insula and the inferior frontal gyrus.
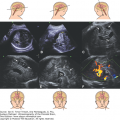
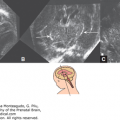
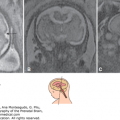
In Figure 15–3, the bilateral common carotid arteries, the ICA and the basilar arteries, were imaged in the coronal plane, as seen on the left side of the figure, while the circle of Willis and two MCAs were demonstrated from the parietal view using 3D power Doppler reconstructed imaging, seen on the right of Figure 15–3, at 12 postmenstrual weeks’ gestation. The ACA and its branches can be demonstrated using the sagittal plane from the late first trimester. From the late first or early second trimester, the MCA, ACA, and their branches can be demonstrated by transvaginal 3D power Doppler (Figure 15–4). In the second trimester, the MCA is also easy visible, and its peak systolic velocity (PSV) values increase from the 22nd to 38th postmenstrual week.12 Figure 15–5 shows the median 2D/3D bidirectional power Doppler images by TVS/transfontanelle sonography of the ACA, callosomarginal artery, and their branches. Figure 15–6 shows serial, consecutive tomographic US images of coronal and sagittal sections of the above arteries. If a more accentuated impression of the brain vessels is sought, 3D rendering will provide those images. These are excellent teaching materials. Figure 15–7 shows such reconstructed 3D angiography of normal cerebral circulation at 31 postmenstrual weeks.
Figure 15–3.
Vascular structure of normal 12 postmenstrual week brain by 3D power Doppler angiographic rendering. (A) Coronal power Doppler image of common carotid arteries (CCA), internal carotid arteries (ICA), and their branches. (B) Axial or horizontal 3D power Doppler angiographic image obtained from the parietal direction. The circle of Willis (asterisk) is clearly visualized. MCA, middle cerebral arteries.

Figure 15–4.
Conventional 3D power Doppler image of fetal brain circulation. (A) Cranial view. The bilateral internal carotid arteries (ICA) and middle cerebral arteries (MCA), as well as the branches of MCA, are demonstrated. (B) Anterior oblique view. The anterior cerebral artery (ACA) and the pericallosal artery (PcA) are demonstrated.

Figure 15–6.
Anterior cerebral artery and its branches in a 24 postmenstrual weeks’ normal brain (median view). (A) Two-dimensional (2D) power Doppler image by B-mode demonstrates the corpus callosum (CC). (B) 3D reconstructed angiographic image. ACA, anterior cerebral artery; CMA, callosomarginal artery; SSS, superior sagittal sinus. The cingular branch of the CMA runs along the cingulate gyrus.

Figure 15–7.
3D tomographic ultrasound (US) image of brain circulation at 31 postmenstrual weeks. (A) Tomographic power Doppler imaging of serial sagittal sections on which the anterior cerebral arteries and their branches are seen. The image at the center represents the perfect median section. (B) Successive and serial coronal sections. The middle cerebral arteries and their branches are seen on these sections.

Tomographic US imaging is useful for obtaining orientation of cerebral vessels. High-frequency TVS employing a 6 to 12 MHz transducer (Voluson E8, GE Healthcare, Waukesha, Wisconsin) has enabled the demonstration of vessels on the cerebral pial surface, as shown in Figures 15–8 and 15–9. Furthermore, the author (RKP) succeeded in demonstrating the fine medullary vessels running from the pial surface toward the subependymal area by bidirectional power Doppler with 3D angiostructural imaging,7,13 as shown in Figure 15–10. As seen on these images, 3D imaging technology with high-frequency TVS and 3D power Doppler allows the images to be assessed on the orthogonal display as well as on the rendered planes. Conventional 3D power Doppler technology could not depict small-caliber blood vessels; however, recent advances in 3D technology using bidirectional power Doppler allows tiny blood vessels to be depicted.
Figure 15–9.
Coronal view of pial vascularity along the cerebral sulci and gyri by 3D bidirectional power Doppler angiography at 33 postmenstrual weeks of gestation. (A) 3D bidirectional power Doppler angiogram of pial vessels along the gyri and sulci. (B) 3D reconstructed image of the cerebral by surface y gray scale. Protrusion of the brain surface due to gyral formation is demonstrated.

Figure 15–10.
Parietal/tangential view of pial vascularity along cerebral sulci and gyri by 3D bidirectional power Doppler at 30 weeks of gestation. (A) 3D reconstructed gray scale image of the cerebral surface. Falx cerebri and gyral formation are well demonstrated. (B) 3D bidirectional power Doppler sonoangiogram of superficial vascularity and pial vessels along the gyri and sulci. SSS, superior sagittal sinus.

Using this technique, medullary vessels are detectable from the early second trimester developing into numerous “showerlike” vessels. Before 20 weeks, as seen in Figure 15–9, the medullary veins are demonstrated as red color or red/blue colors by bidirectional power Doppler, and thereafter remarkably rapid development is seen in the previously mentioned showerlike vessels (Figure 15–11). In the fetal brain, the medullary vessels within the deeper cerebral white matter are more developed than the subcortical veins located within the subcortical white matter.14 The maldevelopment of medullary vessels may indicate developmental abnormalities and may predict subsequent hydrocephaly and postnatal neurologic deficit. At this time we have no scientific proof of the clinical importance of this fascinating display of the fine details of fetal brain circulation. The author (RKP) has been investigating the assessment of medullary veins in normal and abnormal brain structure. It is expected that the investigation will be one of the clues in evaluation of the relations between the fetal brain development and postnatal neurologic findings.
Figure 15–11.
Development of medullary vessels with advanced gestational age. 3D reconstructed coronal power Doppler images from the same fetus at 19 postmenstrual weeks of gestation (A) and 24 postmenstrual weeks (B). At 19 postmenstrual weeks, medullary vessels (M) are immature, and most of the veins run toward the pia mater. With advancing gestational weeks, medullary vessels (M) mature and increase in numbers. The vessels run in the central direction from the cerebral cortex toward the longitudinal caudate vein of Schlesinger (S).

For studying the MCA according to anatomical data, Mari and collaborators15 suggested a plane more caudal to the cerebral peduncles in the section containing the pons and the medulla oblongata and greater paired wings of the sphenoid. It can also be visualized at the level of the cerebral peduncles at its anterolateral border, running anterolaterally toward the lateral edge of the orbit.
The base of the skull at the level of the temporal and sphenoidal bones is the preferred plane for recording Doppler signals from the ACA and PCA. The ACA flow-velocity waveforms can be obtained close to the midline, anterior to the pulsating ICA, and half the distance from the midbrain to the frontal bone. The PCA recordings are done at the level of the transverse cerebral fissure on the side of the midbrain.
Using the transabdominal route, a prerequisite for recording cerebral Doppler signals is that the head is not too deeply engaged in the maternal pelvis.16 Transvaginal scanning of cerebral vessels was recommended by Lewinsky et al.17 The coronal section obtained by this approach showed separate and easily distinguishable images of these arteries, because the ICAs are located medially and inferiorly to the corresponding MCAs. With the use of a transfontanelle approach in the newborn, detection of flow is easily obtained from the ACA, where it curves around the corpus callosum.18 In most studies of the fetal cerebral circulation, Doppler-derived data are gained from the MCA and ICA due to the ease of obtaining recordings. Few studies have reported on data collected from other cerebral vessels (ie, the ACA and PCA).19,20,21 To correlate fetal with neonatal cerebral flow data, the same vessels should be studied.22,23
Detection of vessels is based on visualization of pulsatile flow-velocity waveforms with duplex systems or by using color flow imaging.24 Transducers with carrier frequencies of 2.5, 3.5, and 5 MHz are usually used, the sample gate not exceeding 3 to 4 mm. This allows clear flow-velocity signals without interference from other nearby vessels. A high-pass filter of 50 to 150 Hz is applied to remove signals originating from slow-moving tissues in the path of the Doppler beam. The angle of insonation is kept as small as possible, and the low-power output mode should be used throughout the study. For standard conditions, all samples are taken with subjects in the semi-recumbent position and during fetal apnea, as high-amplitude fetal breathing modulates the blood flow.25
For calculation of qualitative Doppler indices, velocity waveforms are recorded, and peak velocity, end-diastolic velocity, and mean maximum velocity are measured. Three to five consecutive waveforms are analysed, and the results are averaged. Using these Doppler variables, the pulsatility index (PI), defined as the difference between the PSV and end-diastolic value divided by the mean maximum flow velocity,26 may be calculated. Substantial interobserver agreement and intraobserver repeatability were found for cerebral vessels studied.27,28
Other qualitative parameters may be determined but are less frequently used: the ratio of peak systolic to maximum end-diastolic velocity (S/D ratio), the resistance index (RI) (the difference between peak systolic and end-diastolic velocities divided by the PSV), and the cerebral index (PSV minus S/D ratio).29 Ratios of qualitative parameters in other fetal vessels are also in use: the ratio of RI in the fetal common carotid artery to the RI in the umbilical artery, as described by Arabin et al,30 or the ratio between the cerebral RI and the placental RI, as described by Arbeille and colleagues.21 The use of color flow imaging enables accurate measurement of the angle of vessel insonation to determine absolute mean blood flow velocity values in intracranial vessels.31
The venous circulation of the fetal brain can be identified by color Doppler. Recently, the US-anatomical correlates were established for the venous blood flow in the fetal brain, and the reference values for flow-velocity waveforms in the transverse sinus were documented.32
Power Doppler improves the sensitivity of detection of the presence of flow, when compared with conventional color Doppler velocity imaging. Fetal intracerebral arteries and veins that could not have been imaged by the transabdominal approach were demonstrated using a combination of TVS and power Doppler flow mapping.4
The intracranial circulation becomes visible as early as the 8th week of pregnancy, when arterial pulsation can be detected on an axial view of the embryonic skull. In a study by Kurjak and coworkers33 using transvaginal US, the visualization rate of pulsation on the base of the skull increased from 50% at the 8th gestational week to 83% at the 10th week. From the 11th week onward, it became a constant finding. It is very difficult to distinguish blood flow between the various cerebral arteries because the distances are in a range of a few millimeters or even less. The waveform signal profile at this gestational age is characterized by the absence of an end-diastolic component.
During the third trimester, continuous flow is present throughout the cardiac cycle in the ICA, confirming the existence of low peripheral vascular resistance in the fetal brain.34 Flow-velocity waveforms from the fetal MCA are highly pulsatile, and the presence of end-diastolic frequencies becomes more common with advancing gestation.31 Thus, end-diastolic frequencies were present in 75% of fetuses at 18 to 25 weeks and in all fetuses examined after 34 weeks’ gestation.31
The PI of the MCA was found to be higher than that for either the ICA or the proximal ACA.15 Hata et al35 found the RI of the PCA to be lower than that of the MCA and ACA.
These differences emphasize the need for an exact definition of the vessel that is being insonated and could be caused by different resistances in various portions of the cerebral circulation. In vitro examination of the contractile properties of the common carotid artery in the fetal lamb has shown that this vessel has less dilating capacity in response to hypoxia than do the intracranial arteries.36 However, in cross-sectional studies, mean blood flow velocities in the common carotid artery increased throughout pregnancy in contrast to aortic velocities, which tend to decrease toward the end of pregnancy. The PI in the aorta remains constant, whereas in the common carotid artery, it falls steeply after 32 postmenstrual weeks.37 A significant decrease in the PI was also observed in the MCA, especially after 36 weeks (Table 15–1)38,39,40. These results suggest that with advancing gestation there is redistribution of the fetal circulation, with decreased impedance to flow to the fetal brain, presumably to compensate for the progressive decrease in fetal blood PO2. In studies of the MCA in the second trimester,41,42,43 an increasing PI was found until the late second trimester, followed by a decline in the third trimester. Mari and Deter43 attributed the low PI values at the beginning and end of pregnancy to increased metabolic requirements and therefore lower cerebral vascular impedance to blood flow.
Studies of waveforms recorded from the fetal ICA demonstrated that the PI remains fairly constant during the last trimester of pregnancy, and only during the last 4 weeks does there seem to be a slight decrease.31 Reference RIs of the fetal MCA were established in a large and minimally selected population attending a single clinic.44 Cerebral vascular resistance decreases constantly up to gestational week 42.44
In a longitudinal study,22 fetal and neonatal cerebral blood flow velocities were assessed in the MCA in 40 uncomplicated pregnancies during the third trimester and in 22 neonates born from these pregnancies (Table 15–2). PSV, temporal mean, and end-diastolic flow velocities increased during the third trimester and were significantly higher from 36 weeks’ gestation on, as compared with values obtained at 28 postmenstrual weeks, suggesting an increase in actual blood flow. The PI and RI of the MCA did not differ significantly during this period. Immediately after birth, flow velocities decreased significantly and remained lower during the first 5 postnatal days compared with fetal values. The PI and RI of the MCA tended to decrease during the first postnatal day but stabilized afterward. These alterations in cerebral blood flow in the transition from the fetal to the neonatal state are explained by local rather than central cardiovascular changes, mainly the local effect of oxygen on peripheral vessels.45
| Vascular Index | 26 to 27 Weeks* | 40 Weeks* | References |
|---|---|---|---|
| Common carotid artery pulsatility index | 2.13 ± 0.11 | 1.89 ± 0.07 | 37 |
| Internal carotid artery pulsatility index | 1.63 ± 0.35 | 1.31 ± 0.41 | 33 |
| Middle cerebral artery | |||
| Pulsatility index | 2.30 ± 0.48 | 1.82 ± 0.38 | 31 |
| Systolic/diastolic ratio | 6.89 ± 1.48 | 4.23 ± 0.67 | 68 |
| Resistance index | 0.93 ± 0.049 | 0.68 ± 0.087 | 35 |
| Mean velocity (cm/sec) | 5.3 ± 2.3 | 11.3 ± 3.1 | 31 |
| Anterior cerebral artery resistance index | 0.83 ± 0.05 | 0.79 ± 0.04 | 172 |
| Posterior cerebral artery resistance index | 0.73 ± 0.05 | 0.70 ± 0.06 | 118 |
The physiologic changes to cerebral blood flow in normal pregnancy are summarized in Table 15–3.
| State | Impedance | References | ||||
|---|---|---|---|---|---|---|
| Internal Carotid Artery | Middle Cerebral Artery | Anterior Cerebral Artery | Posterior Cerebral Artery | Mean Blood Velocity | ||
| Gestational age | ↓ | N/↓ | ↓ | ↓↓ | ↑ | 31, 33, 37, 39, 46, 47 |
| ↑ Fetal heart rate | ↓ | — | — | — | — | 49 |
| ↓ Fetal heart rate | ↑ | — | — | — | — | 48, 49 |
| Fetal breathing movement | ↑/↓ | — | — | — | — | 50 |
| Fetal behavior stage 2F | ↓ | — | — | — | — | 52 |
| Plasma glucose concentration | ↑ | ↓/↑a | — | — | — | 53, 54, 56 |
| Fetal head compression | ↑ | ↑ | — | — | — | 57 |
| Uterine contractions | ↑/N | ↑/↓ | — | — | — | 60,61,62 |
| Fetal anemia | ↑/N | ↑/N | — | — | ↑ | 64, 65 |
| Oilgohydramnios | ↑ | ↑ | — | — | — | 58, 90,91,92,93,94 |
| Growth retardation | ↓↓ | ↓↓/↓ | ↓ | ↓ | ↑ | 15, 17, 21, 29, 31, 43, 68, 69 |
| Fetal hydrocephaly | ↑ | ↑/N/↓ | — | — | — | 118,119,120 |
| Arteriovenous malformation | — | ↓ | — | — | — | 156–157 |
| ↑ Pco2 | ↓ | ↓ | — | — | — | 133, 134 |
| ↓ Pco2 | ↓ | ↓ | — | — | ↑ | 31, 69 |
An inverse correlation was found between fetal heart rate and PI in the MCA of fetuses with heart rate decelerations48 and with tachycardia secondary to ritodrine infusion.49 Within the normal range of fetal heart rate, Doppler indices did not alter significantly.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree