Since first being performed in 1986, fetal MRI has rapidly become a standard of care for the secondary evaluation of fetal abnormalities involving the central nervous system (CNS) and thoracic and abdominopelvic cavities. Fetal MRI is most commonly performed for the evaluation of the fetal CNS when prenatal ultrasound demonstrates an abnormality. This chapter will provide an overview of fetal MRI by addressing foundations of fetal neuroimaging followed by a case-based approach to various disease entities of the brain and spine.
Fetal Central Nervous System Magnetic Resonance Imaging Techniques
At What Gestational Age Is Fetal Central Nervous System Magnetic Resonance Imaging Best Performed?
There are no known fetal contraindications to MRI at any gestational age. However, fetal CNS MRI before 20 weeks of gestation is of limited diagnostic value mainly because of the small size of the fetus and the noncompletion of organogenesis. Furthermore, fetal motion at such an early gestational age, even with single-shot techniques, makes imaging extremely difficult. Therefore, the majority of fetal CNS MRI is recommended at or after 20 weeks gestational age. Thereafter, fetal imaging should occur at a time point that not only best answers the question at hand but also provides information for management of the pregnancy. Most severe CNS malformations can be seen by 20 weeks of gestation. For these reasons, fetal CNS MRI commonly occurs between 20 and 24 weeks. It should be remembered, however, that there are diagnoses that may not be seen at such an early gestational age, including such things as cortical malformations that are focal or regional, gray matter heterotopia, subependymal nodules in tuberous sclerosis, and split cord malformations, among many others. Other times, it may be acceptable to both the patient and the ordering physician that fetal CNS MRI be performed late in the third trimester. For example, a fetus with mild ventriculomegaly for which expectant management is desired may be imaged late in the third trimester to then obviate the need for postnatal MRI. At other times, it may be critical for a fetus to be imaged late in the pregnancy just before delivery, for example, a fetus with a neck mass that warrants its evaluation with respect to the airway and for which an ex utero intrapartum treatment procedure is being contemplated. Other indications include maternal and/or fetal trauma, expected postnatal instability that would thwart a postnatal MRI and other expectant perinatal delivery management. Fetal MRI for CNS abnormality is usually not repeated during the pregnancy after the initial diagnostic fetal MRI, unless perinatal decisions would be affected, such as the one just described.
What Are Common Indications for Fetal Central Nervous System Magnetic Resonance Imaging?
The most common indication for fetal MRI is for the evaluation of cerebral ventriculomegaly. Under this premise, the primary clinical question is whether the ventriculomegaly is isolated (without identifiable cause or additional abnormalities) or nonisolated (with an underlying cause, such as aqueductal stenosis or additional abnormalities detected). Suspected corpus callosal abnormalities are a common indication, with the cavum septum pellucidum being an easily detected structure, and a marker of callosal development, on fetal ultrasound. Evaluation of the posterior fossa is also a common indication for fetal CNS imaging, which primarily involves evaluation of cerebellar development. Vascular abnormalities, including hemorrhage, malformations, and vascular insults, particularly as a complication of monochorionic twin pregnancies, are additional indications for fetal CNS evaluation. Fetal spine imaging typically serves to evaluate neural tube defects, scoliosis, and occasionally mass lesions, such as sacrococcygeal teratomas. Finally, evaluation of head and neck masses, especially their relation to the airway, encompass a small group of patients undergoing fetal imaging. An important point to make is that fetal MRI is indicated only when ultrasound adjunctive information is sought and when such information will have meaningful implications for patient care or counseling. Such implications include pregnancy management, mode or location of delivery, expected perinatal resuscitation needed (e.g., ex utero intrapartum treatment procedure), and parental education to include more accurate prognostication and to ultimately address parental anxiety.
What Protocol Should Be Used to Evaluate Fetal Central Nervous System Abnormalities?
The most important initial step in performing a successful fetal MRI after patient positioning is the ideal placement of the coil. The initial three-plane localizer should be used as a quick estimate of coil positioning with ideal placement resulting in clear visualization of the entirety of the gestational sac, preferably centered on the CNS structure in question. It is advisable that the radiologist be in a supervision position at the console or from the reading room as the technologist is performing the examination to evaluate the need for repeat sequences.
Standard protocols should exist for fetal imaging, including at least a body and CNS protocol. An example of a standard CNS protocol is listed in Table 24.1 .
| Sequence | Plane | TR (ms) | TE (ms) | Voxel Size (mm) | Comment |
|---|---|---|---|---|---|
| Maternal gestational sac localizer | Axial, sagittal, coronal | 6.7 | 2.95 | 1.8 × 1.8 × 7.0 | Entire uterus/pelvis |
| T2 HASTE | Axial | 700 | 133 | 1.0 × 1.0 × 7.0 | Entire uterus/pelvis |
| T1 FLASH | Axial | 125 | 1.86 | 1.3 × 1.3 × 7.0 | Entire uterus/pelvis |
| Fetal TrueFISP localizer | Axial, sagittal, coronal | 3.2 | 1.6 | 3.5 × 3.5 × 7.0 | Entire fetus |
| Fetal brain localizer | Axial, sagittal, coronal | 3.2 | 1.6 | 3.5 × 3.5 × 2.0 | Fetal brain |
| T2 HASTE | Axial, sagittal, coronal | 1000 | 133 | 1.0 × 1.0 × 3.0 or 1.0 × 1.0 × 2.0 | Fetal brain |
| T1 FLASH | Axial | 110 | 2.5 | 1.2 × 1.2 × 4.0 | Fetal brain |
| DWI/ADC MAP | Axial | 5500 | 129 | 2.0 × 2.0 × 4.0 | Fetal brain |
| Spine localizer | Axial, sagittal, coronal | 3.2 | 1.6 | 3.5 × 3.5 × 7.0 | Fetal spine |
| T2 HASTE | Sagittal | 1000 | 133 | 1.0 × 1.0 × 3.0 | Fetal spine |
| TrueFISP | Sagittal | 3.90 | 1.95 | 1.0 × 1.0 × 3.0 | Fetal spine |
| T2 HASTE | Axial, coronal | 1000 | 133 | 1.0 × 1.0 × 3.0 | Fetal abdomen and thorax |
| T1 FLASH | Sagittal | 3.9 | 1.95 | 1.3 × 1.3 × 5.0 | Fetal abdomen and thorax |
Fetal Central Nervous System: Normal Development
What Is the Initial Approach to Evaluating the Fetal Brain on Magnetic Resonance Imaging?
The initial approach to all fetal MRI is evaluation of the maternal anatomy, including evaluation of placenta location (and with twins, chorionicity and amnionicity), the subjective amount of amniotic fluid, the presence of a two- or three-vessel cord, gross evaluation of the cervix, and finally the evaluation of extrauterine pathology of the maternal pelvis and spine.
Next, attention to the fetal anatomy is warranted. Before evaluating the brain parenchyma, it is important to first determine situs. Although the initial temptation is to identify the cardiac apex, the liver, and stomach to decide on brain sidedness, this should not be performed because a fetus may have situs inversus. Instead, it is best to determine by orienting to the fetus in three dimensions. By determining whether the fetus is breech or cephalic in presentation and on which side of the maternal pelvis the fetus lies, the radiologist can determine situs.
Evaluation of the brain then involves a thorough biometric evaluation of the fetal brain. Biometric evaluation includes measurement of the cerbral biparietal diameter (BPD), bone parietal diameter, frontooccipital diameter (FOD), corpus callosal length (AP length).
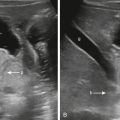
Additional biometrics that should be performed include the greatest transverse dimension of the cerebellum measured in the axial plane, the transverse cerebellar dimension (TCD). The cisterna magna (CM) should also be measured and should not exceed 10 mm. Measurements of the TCD and CM are shown in Figure 24.1 . Other biometrics include cranial caudal and AP measurements of the vermis and pons. Nomograms for these measurements are readily available in definitive fetal textbooks. Beth Kline Fath et al. Fundamental and Advanced Fetal Imaging Ultrasound and MRI 2015).

And finally, the lateral ventricles are measured typically in the coronal plane at its maximal transverse width at the level of the atrium. Coronal measurements have been shown to be highly concordant with axial measurements of ultrasound. Lateral ventricles less than 10 mm are considered normal. Ventricles between 10 and 12 mm represent mild ventriculomegaly, between 12 and 15 mm moderate ventriculomegaly, and greater than 15 mm severe ventriculomegaly. Some authors simplify this scheme and classify mild ventriculomegaly as ventricles between 10 and 15 mm and severe ventriculomegaly as greater than 15 mm. The third and fourth ventricles are not typically quantitatively measured, although both should be qualitatively assessed as being normal or enlarged.
The initial evaluation of the fetal brain is not complete until the gyral/sulcal pattern of the brain is evaluated and compared with expected for the given gestational age. Although a detailed discussion of normal gyration and sulcation as a function of gestational age is beyond the scope of this chapter, there exists multiple excellent atlases on normal fetal MRI brain anatomy. Both the experienced and inexperienced fetal imager benefit from comparative evaluation of case patients with reference images to ensure normal cortical development. Focal or regional cortical malformations remain among the most difficult diagnoses to make in utero, particularly until the mid- to late third trimester.
Ventriculomegaly
As previously noted, ventriculomegaly is the most common indication for fetal MRI. After deciding that ventriculomegaly is indeed present, the primary objective of the radiologist is to decide whether the ventriculomegaly is isolated, in that no other intracranial abnormalities are present, or nonisolated, in that causative or additional abnormalities are present. It is important to remember that the diagnosis of isolated ventriculomegaly on imaging is a diagnosis of exclusion. However, it does not imply that a causative factor is absent. It simply indicates that the cause is not identified on imaging. Of course a causative factor may become apparent later in pregnancy, postnatally, or even later in childhood. Table 24.2 lists common causes of nonisolated ventriculomegaly.
Congenital Malformation
|
Destructive
|
What Is the Value of Fetal Magnetic Resonance Imaging in Evaluating Ventriculomegaly?
Numerous studies have evaluated the ability of MRI to offer additional findings compared with ultrasound and, more importantly, offer additional findings that result in a change in management. Such a change in management often is a result of MRI to offer a more exact diagnosis than can be offered by ultrasound alone. For example, obstructive ventriculomegaly can be confirmed to be secondary to aqueductal stenosis or associated with a syndrome such as Walker-Warburg. These additional findings may change management, modify the mode or location of delivery, or alter the anticipatory perinatal care of the fetus, including neurosurgical intervention. Another important point is that even when MRI does not detect additional abnormalities, in many cases it affords confidence in the correct diagnosis initially made by ultrasound.
How Do I Decide if the Ventriculomegaly Is Obstructive or Nonobstructive?
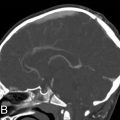
Deciding whether ventriculomegaly is obstructive or nonobstructive is a logical initial step that helps hone the differential. First, evaluations of which ventricles are involved can be very helpful. The overwhelming majority of obstructive processes occur at the level of the posterior fossa beginning with the cerebral aqueduct. Therefore significant third ventricle dilatation is a strong clue that an obstructive process may be present. Then, attention should be placed on the subarachnoid spaces overlying the supratentorial convexities. Effacement of the cerebral convexities is a strong clue that an obstructive process is present. This often coexists with thinning of the cortical mantle and associated effacement of age-appropriate cerebral sulci, such as the sylvian fissure. And finally, the presence of macrocephaly can be another clue that an obstructive process is present ( Fig. 24.2 ). Two important points should be made. Fetuses with Chiari type II malformation do not typically have macrocephaly in contradistinction to obstructive processes, such as aqueductal stenosis (see Fig. 24.2 ). Note the presence of CSF effacement in Chiari type II malformation due to intracranial hypotension. And finally, it is important to remember that although macrocephaly is often secondary to obstructive hydrocephalus, there are rare instances where megalencephaly, defined as enlargement of the brain parenchyma itself, is the primary cause of macrocephaly. Fetuses with megalencephaly may have diffuse supratentorial cortical dysplasia as can be seen with perisylvian syndromes. Ventriculomegaly may coexist with megalencephaly but may not be obstructive in origin.

Is the Corpus Callosum Normal?
Abnormalities of the corpus callosum are a common cause of ventriculomegaly and should be the initial evaluation in patients with nonobstructive ventriculomegaly. The corpus callosum is embryologically completely formed by 20 weeks of gestation and therefore is identifiable at the onset of when fetal MRI is typically offered. Evaluation of the corpus callous heavily relies on the multiplanar capabilities and superb resolution of MRI ( Fig. 24.3 ). It cannot be stressed enough that proper evaluation of the corpus callosum must include thin-slice, preferably 2 mm skip 0 mm sagittal T2-weighted imaging, and that the sagittal sequence must be perfectly aligned. Also acceptable is 3-mm slice thickness, but one may still find some difficulty in visualizing the corpus callosum completely at early gestational ages. It is not uncommon that initial attempts at performing a sagittal sequence be plagued by mild obliquity because of constant or inconstant fetal motion. The technologist should make reasonable attempts to perform the perfect sagittal imaging and of course should recognize when such an image has been successfully obtained (see Fig. 24.3 ). Ideally the corpus callosum should be imaged in its entirety, but as noted, even with perfect sagittal imaging this may be difficult. Identification of the C-shaped corpus callosum is necessary, including the splenium, to interpret as normal. Complete evaluation of the callosum also relies on visualizing the callosum on axial images and coronal images and identification of the cavum septum pellucidum. Coronal images show the cavum septum pellucidum and the commissural fibers of the corpus callosum especially well. If there is agenesis of the corpus callosum, Probst bundles and the characteristic “longhorn” dysmorphology of the lateral ventricles are well seen in the coronal plane. Axial images are also helpful in identifying the normal cavum septum pellucidum or colpocephaly or “teardrop” configuration of the posterior ventricles in agenesis or severe hypogenesis of the corpus callosum ( Fig. 24.4 ). Mild hypogenesis of the corpus callosum, however, can be difficult to evaluate in some cases, but is especially difficult on axial and coronal images because of suboptimal evaluation of the polar segments of the corpus callosum. Corpus callosum biometry with normogram reference is thus especially important for all fetuses.


Notably, in rare instances, agenesis of the corpus callosum may coexist with interhemispheric cysts that either communicate or do not communicate with the lateral ventricles. In cases in which agenesis of the corpus callosum is associated with cysts that communicate with a ventricular system obstruction at the cerebral aqueduct is present. These cysts are diverticula of the ventricular system (see Fig. 24.4 ).
Finally, agenesis and hypogenesis of the corpus callosum can occur in isolation and nonisolation. Numerous syndromic and nonsyndromic diagnoses are associated with abnormalities of the corpus callosum.
Are the Right and Left Cerebral Hemispheres and the Central Gray Matter Fully Divided?
Holoprosencephaly is defined by a lack of complete division of the respective cerebral hemispheres of varying severity. The mildest form, referred to as lobar holoprosencephaly, has the most complete division from the contralateral hemisphere with only partial fusion at the level of the frontal lobes often with falcine hypoplasia. This is in contradistinction to the alobar subtype, where there is essential lack of differentiation between the right and left brain. Alobar holoprosencephaly is in fact the most common form of holoprosencephaly seen in the prenatal period. Such fetuses have a large “pancake”-shaped brain in the anterior cranial fossa with a large monoventricle and dorsal cyst. The corpus callosum and falx are absent, and the central gray matter and hypothalamus are fused ( Fig. 24.5 ). Semilobar holoprosencephaly exists between the continuum of lobar and alobar holoprosencephaly. It should be noted that the distinction between the respective subtypes of holoprosencephaly is not concrete, and therefore the fetal imager should not be overly concerned with distinguishing a severe semilobar from alobar holoprosencephaly, for example. More importantly, the fetal imager should ensure high-quality 2- to 3-mm axial and coronal T2-weighted imaging to be able to assess for milder cases of holoprosencephaly. This includes close scrutiny for frontal lobe fusion in lobar holoprosencephaly and fusion of the superior frontal and parietal lobes in the middle interhemispheric variant of holoprosencephaly, also referred to as syntelencephaly. And finally, fusion of the diencephalon at the level of the thalamus may easily be missed without close scrutiny of this region. In isolated diencephalic fusion, ventriculomegaly may be seen. Agenesis of the corpus callosum and interhemispheric cysts may also be present. Scrutiny of this region is also important in evaluation of diencehalic mesencephalic junction dysplasia (Severino M, Righini A, Tortora D, Pinelli L, Parazzini C, Morana G, Accorsi P, Capra V, Paladini D, Rossi A. MR Imaging Diagnosis of Diencephalic-Mesencephalic Junction Dysplasia in Fetuses with Developmental Ventriculomegaly. AJNR Am J Neuroradiol. 2017 Aug;38(8):1643-1646. doi:10.3174/ajnr.A5245 . Epub 2017 Jun 8. PMID: 28596193 ; PMCID: PMC7960408.)

Is the Cavum Septum Pellucidum Present?
Identification of the cavum septum pellucidum is an extremely important exercise in evaluating the fetal brain. As with other parts of the fetal brain, high-quality, high-resolution fetal brain imaging is critical in identifying the cavum septum pellucidum and any associated abnormalities. It is best identified on coronal images as two separate thin leaflets coursing from the corpus callosum to the fornix partitioning the right from the left lateral ventricle.
Identification of a normal septum pellucidum is important because in greater than 95% of cases, absence of the septum pellucidum occurs as a nonisolated abnormality. Absence of the septum pellucidum is seen with callosal agenesis, with presence of the septum pellucidum arguing against callosal agenesis. Furthermore, absence of the cavum septum pellucidum may be seen with a variety of congenital abnormalities, including holoprosencephaly, septo-optic dysplasia, and bilateral schizencephaly, among many others.
One especially important note should be made. With chronic severe hydrocephalus the septum pellucidum may be absent. In such cases, however, the septum pellucidum is usually absent as a function of acquired fenestration with primary agenesis less common.
Are There Small or Large Portions of the Lobar Cerebral Brain Parenchyma That Are Not Present?
Porencephalic cysts or regions of porencephaly are areas of brain parenchyma that have undergone some form of injury. It is not uncommon for such regions of injury to be wedge shaped, and by definition such regions are isointense to cerebrospinal fluid (CSF) on all sequences. The cause of porencephalic cysts is thought to vascular in nature, posttraumatic or posthemorrhagic, or even secondary to infection. Notably, ischemia of varying severity and of various organs, including the brain, may occur in the living fetus after singular fetal demise in a monochorionic pregnancy ( Fig. 24.6 ). The resulting injury to the remaining fetus may be life-threatening.