(1)
Princess Elizabeth Hospital Le Vauquiedor St. Martin’s Guernsey, Channel Islands, UK
(2)
Brighton and Sussex Medical School, Brighton, England UK
Abstract
The fibroadenoma is a benign tumour occurring most commonly in the second and third decades (Foster et al. 1988), becoming infrequent in postmenopausal women. In young patients, fibroadenomas tend to be cellular and the cellularity declines with age. Increase in cellularity can also be present in postmenopausal women, which has been attributed to a response to the unopposed action of oestrogens (Foster et al. 1988). Fibroadenomas commonly present as palpable lesions, but some tumours regress with age, and in postmenopausal women, they can be detected mammographically with or without associated calcification.
Learning Points
Fibroadenomas and phyllodes tumour are collectively referred to as fibroepithelial tumours.
Due to increased stromal cellularity, it is not always possible to differentiate a cellular fibroadenoma from a phyllodes tumour on needle core biopsy.
Complex fibroadenomas are associated with an increased risk of subsequent breast cancer.
Molecular evidence suggests that fibroadenomas are polyclonal therefore hyperplastic lesions and not neoplastic.
Observational and molecular studies suggest fibroadenomas can transform into phyllodes tumours.
Genetic abnormalities in both the stromal and epithelial components of fibroadenomas suggest the epithelium is potentially neoplastic.
Phyllodes tumours are graded as benign, borderline and malignant.
Fibroadenomatoid hyperplasia may be a precursor of multiple fibroadenomas.
As there are no pathognomonic features of a hamartoma, the final diagnosis may be made on needle core biopsy.
8.1 Fibroadenoma
8.1.1 Clinical Features of a Fibroadenoma
The fibroadenoma is a benign tumour occurring most commonly in the second and third decades (Foster et al. 1988), becoming infrequent in postmenopausal women. In young patients, fibroadenomas tend to be cellular and the cellularity declines with age. Increase in cellularity can also be present in postmenopausal women, which has been attributed to a response to the unopposed action of oestrogens (Foster et al. 1988). Fibroadenomas commonly present as palpable lesions, but some tumours regress with age, and in postmenopausal women, they can be detected mammographically with or without associated calcification.
Hunter et al. (1996) reviewed the occurrence of fibroadenomas in postmenopausal women who were referred for biopsies at the Tucson Breast Center, Arizona. A total of 100 fibroadenomas were reported in 709 breast biopsies. Fifty-two of the fibroadenomas were in premenopausal women and 44 in postmenopausal women. Eleven of the 44 postmenopausal women reported hormone use. Fibroadenomas constituted 20 % (39/195) of benign masses and 12 % (39/339) of all masses in postmenopausal women and 10 % of all the biopsies (44/447) in postmenopausal women, which included abnormal calcifications or other lesions. Not all fibroadenomas in postmenopausal women were calcified. This study highlights the fact that fibroadenomas are common breast lesions in postmenopausal women who also fall into the screening age group. The importance of accurate radiological assessment of fibroadenoma is to exclude well-circumscribed malignant tumours such as mucinous carcinoma, medullary carcinoma, intracystic papillary carcinoma, sarcoma, phyllodes tumours, metastatic deposits, or lymphoma.
The incidence of fibroadenomas is variable depending on the publication. Dent et al. (1988) reported the prevalence of fibroadenoma to be 7–13 % in women attending breast clinics. Franyz et al. (1951) reported the occurrence of fibroadenoma in 9 % at post-mortem examination. Overall fibroadenomas comprise 50 % of all breast biopsies and the rate increases to 75 % for biopsies in women under the age of 20 (Dent and Cant 1989). Fibroadenomas are more common in black than Caucasian women (Funderburk et al. 1972). Yu and colleagues (1992) assessed the risk factors of fibroadenoma in a case–control study involving 117 fibroadenomas in Adelaide, (Australia) in patients seen between 1983 and 1985. A high Quetelet index (body mass index [BMI]) was associated with reduced risk of developing a fibroadenoma. Age at menarche and age at menopause had no effect on the occurrence of fibroadenomas. The risk of fibroadenomas decreased with the number of full term pregnancies but was increased with use of oral contraceptives at an early age (under 20 years). Alcohol intake and dietary fat intake were not associated with risk increased of fibroadenomas, whereas cigarette smoking and daily vitamin C intake reduced the risk of fibroadenomas.
A fibroadenoma usually presents as a discrete, non-tender mobile lump, (breast mouse) 1–2 cm in diameter and can arise anywhere in the breast. In 10–20 % of the patients, they have two to four tumours in the same breast. These multiple fibroadenomas can present simultaneously or present at different stages over a period of months to years (Williamson et al. 1993). Multiple fibroadenomas have been reported in families raising the possibility of familial predisposition (Haagensen 1971; Nigro and Orgen 1976; Naraynsingh and Raju 1985). Carney’s syndrome is characterised by the presence of cardiac myxomas, spotty pigmentation, endocrine psammomatous melanotic schwannomas and myxoid fibroadenomas (Carney and Toorkey 1991). When Carney and Tooley reported this condition in 145 patients, 31 (21 %) had breast lesions. The breast lesions consisted of accumulation of myxoid material in single lobules, a collection of lobules and in fibroadenomas (myxoid fibroadenomas). The myxoid breast lesions were multicentric and bilateral in eight patients (38 %). The breast lesions were the presenting symptoms as part of Carney’s syndrome in six patients (19 %). The authors advised the clinicians to consider evaluation of the patient and family for the syndrome if a myxoid fibroadenoma is reported pathologically. Carney syndrome is inherited as an autosomal dominant condition and the gene was mapped to chromosomes 2p16 and 17q22-24 (Kischner et al. 2000a). The gene PRKAR1A encodes for type 1A regulatory subunit of protein kinase A (PKA) is a tumour suppressor gene on chromosome 17 and is mutated in Carney complex (Kischner et al. 2000b). In nonfamilial setting, myxoid degeneration causes enlargement of biopsy-proven fibroadenomas in patients on follow-up leading to excision, on suspicion of malignancy (Yamaguchi et al. 2011).
When a fibroadenoma presents as a large mass more than 5 cm in diameter or weighing ≥500 g, the term giant fibroadenoma is applied (Dent and Cant 1989). Giant fibroadenomas affect both adolescent girls and mature women. In mature women, giant fibroadenomas arise in pregnant or lactating women. Because giant fibroadenomas tend to affect adolescent girls more than mature women, the terminology giant fibroadenoma is often used synonymously with juvenile fibroadenoma. A conventional fibroadenoma can also arise in adolescent girls. However, when a giant fibroadenoma arises in an adolescent girl, the tumour grows rapidly, causing breast asymmetry and discomfort (Matz et al. 2013). This requires appropriate surgical skills to ensure satisfactory cosmesis in a young woman.
8.1.2 Mammographic Features of Fibroadenoma
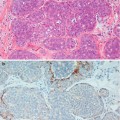
Both symptomatic (Fig. 8.1) and screen-detected fibroadenomas (Fig. 8.2) exhibit a well-circumscribed, round or oval image with or without lobulation on mammography. The mass may be characteristically demarcated from the surrounding tissue to create a halo which indicates benignity (Fig. 8.3). The halo sign is caused by a narrow radiolucent rim around the periphery of a lesion (Tabár and Dean 1985). Exceptionally, intracystic carcinoma or carcinoma arising in a fibroadenoma may give rise to a halo sign. The halo sign is reported to be present in 98 % of fibroadenomas (Sickles 1994). It can also differentiate fibroadenomas from malignant tumours such as medullary carcinoma (Lanyi 1986). Older fibroadenomas can become irregular and ill defined with or without calcification (Fig. 8.4). The calcification tends to be heterogeneous and the following patterns have all been reported in fibroadenoma: complete calcification of the fibroadenoma; coarse, popcorn-like bizarre calcification; evolving calcification, which can be linear, punctate, granular or pleomorphic (Heywang-Köbrunner et al. 2001). Partial calcification with an oyster-shell configuration and crescent-shaped appearance can be present if only part of the capsule is involved (Lanyi 1986).
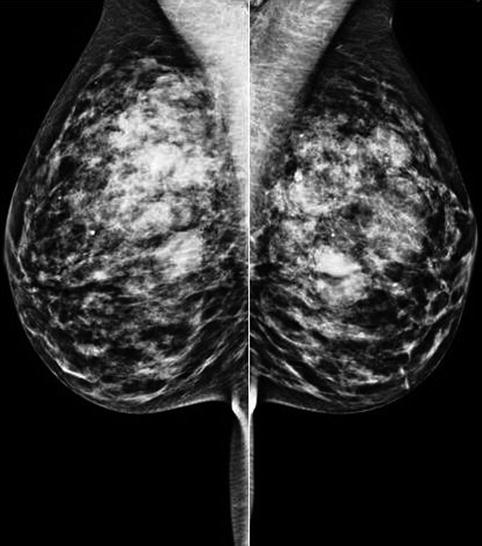
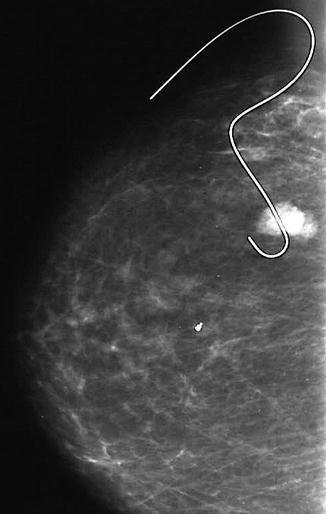
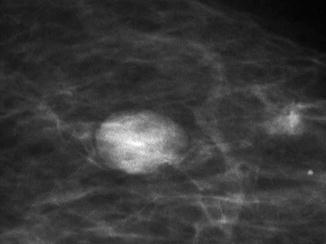
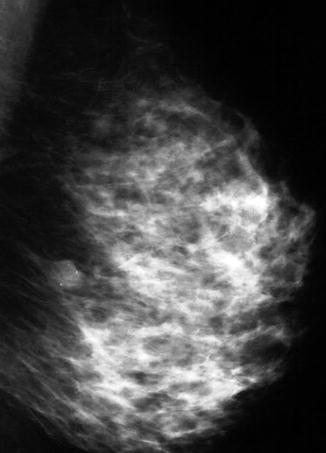

Fig. 8.1
The mammogram of a patient who presented with bilateral and multiple fibroadenomas. Despite the dense breasts, the fibroadenomas can be clearly delineated as well-circumscribed soft tissue masses. The patient also had cysts which were highlighted on ultrasound

Fig. 8.2
This mammogram shows a screen-detected fibroadenoma with a guide wire in situ prior to excision

Fig. 8.3
A dark rim around this screen-detected fibroadenoma is the halo sign which indicates benignity

Fig. 8.4
Long-standing fibroadenomas calcify which can be detected mammographically
8.1.3 Sonographic Features of Fibroadenoma
Ultrasound is useful in assessing well-circumscribed tumours to differentiate solid from cystic lesions. Furthermore, fibroadenomas are more prevalent in young women with dense breasts which can render the fibroadenoma mammographically occult. A fibroadenoma is more easily outlined using ultrasonography in dense breast tissue than in a fatty background (Zonderland 2002). Some fibroadenomas cannot be detected by ultrasound because they are isoechoic with the surrounding tissue. The ultrasonographic appearances of a fibroadenoma include a well-defined round or oval lesion (Fig. 8.5) with a lobulated contour and homogeneous internal echoes (Jackson et al. 1986). In older patients the fibroadenomas tend to become hyalinised with or without calcification and this often produces a heterogeneous appearance on ultrasound (Fig. 8.6). In a hyalinised fibroadenoma, the lesion exhibits weak acoustic shadowing with partial obliteration of the posterior border. Acoustic shadowing becomes more prominent in calcified fibroadenomas.
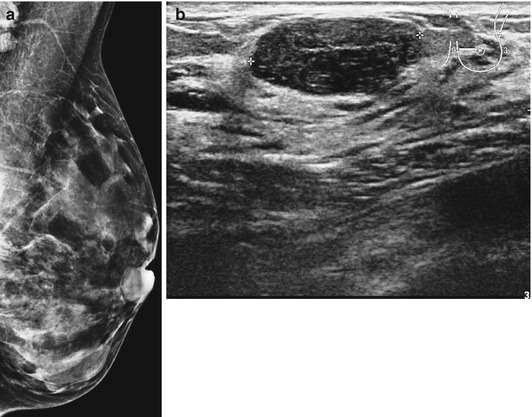
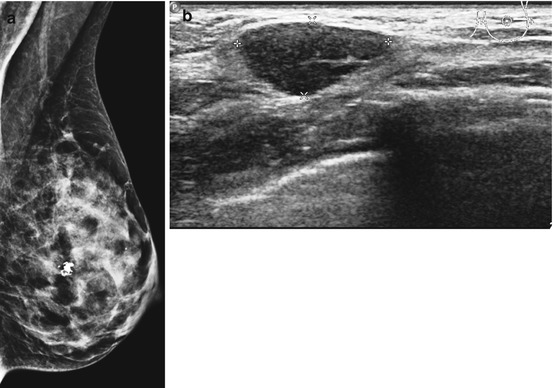

Fig. 8.5
(a) Dense breast in a patient who presented with lump which was mammographically occult. (b) Ultrasound outlined a well-circumscribed fibroadenoma confirmed on needle core biopsy

Fig. 8.6
(a) Screen-detected fibroadenoma with irregular calcification in a dense breast. (b) The ultrasound revealed a well- circumscribed lesion confirmed as fibroadenoma on biopsy
Typical ultrasonic features of fibroadenomas are present in only 20–30 % of cases (Heywang-Köbrunner et al. 2001). Skaane and Engedal (1998) carried out a prospective study of 142 women with fibroadenomas and 194 women with invasive ductal carcinoma to determine the predictive power of sonography in differentiating fibroadenomas from invasive carcinomas. The parameters studied included shape, contour, echo texture, echogenicity, sound transmission and the surrounding tissue related to the tumours. The authors reported that irregular shape and contour, extensive hypoechogenicity, shadowing, echogenic halo and distortion of surrounding tissue were features highly predictive of malignancy. A thin echogenic pseudocapsule was an important predictive feature in benign solid masses. Echo texture was of little value in the differentiation of benign from malignant tumours. The study reported significant overlap of sonographic features in benign and malignant tumours. In an earlier study, Jackson and colleagues (1986) reported that the ultrasound diagnosis of fibroadenoma was correct in 50 out of 76 (65.8 %) histologically confirmed fibroadenomas. The classic features of fibroadenoma with smooth, round or oval mass with homogeneous internal echoes were present in only 12 fibroadenomas. Retrospective review revealed 14 fibroadenomas, which were not visible on ultrasound. The majority of the fibroadenomas had one or more atypical features such as irregular border, lobulation, inhomogeneous internal echo texture or posterior shadowing. Four masses, which were sonographically compatible with fibroadenomas, turned out to be carcinomas. This highlights the necessity of histological diagnosis in all breast masses.
8.1.4 Magnetic Resonance Imaging of Fibroadenoma
Magnetic resonance (MR) imaging is being used increasingly as an adjunct to mammography and ultrasound to differentiate benign from malignant lesions. Young fibroadenomas are highlighted by MR as well-circumscribed focal enhancement with associated non-enhancing internal septations that correspond pathologically to the fibrous bands (Heywang-Köbrunner and Boetes 2002). However, fibrotic fibroadenomas do not enhance or do so only minimally. Therefore, MR is more sensitive in differentiating fibrotic fibroadenoma from malignant lesions than young cellular fibroadenomas from malignant lesions, because of increased enhancement in the latter lesions (Heywang-Köbrunner et al. 2001). Hochman et al. (1997) investigated the histopathological features of fibroadenomas in relation to MR images. They assessed 23 fibroadenomas in 21 patients aged 23–66 years. The fibroadenomas were examined with gadolinium-enhanced MR and were graded for signal intensity, contrast material enhancement, shape and internal septations. The results were correlated with the histopathological findings. Eleven fibroadenomas demonstrated signal intensity without enhancement, and low-signal intensity without enhancement was noted in nine fibroadenomas. Low-signal intensity and lack of enhancement were associated with more sclerotic stroma and older patients. Nineteen out of 23 fibroadenomas (83 %) were lobulated, oval or round. Internal septations were identified in nine out of 14 fibroadenomas (64 %). The study demonstrated varied MR images of fibroadenoma, and this limited the ability to distinguish between benign and malignant masses on the basis of signal intensity and enhancement alone. Lobulation and internal septations, which reflect the intrinsic growth pattern of the fibroadenomas on MR, were more reliable as discriminatory features.
8.1.5 Pathology of Fibroadenoma
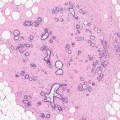
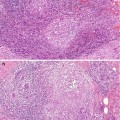
The diagnosis of a fibroadenoma can be made on FNAC or needle core biopsy. On FNAC, the smears contain benign epithelial cells with two cell types, i.e. dark-staining myoepithelial cells and light-staining luminal cells (Fig. 8.7). In the background there are bare nuclei without cytoplasm and these are myoepithelial cells. Cytology can be the definitive diagnosis in adolescent or young women as the fibroadenomas are invariably cellular and with a low risk of false-negative diagnosis. As fibroadenomas are known to regress, conservative management is preferable in young woman. In the older woman or screen-detected fibroadenoma, a needle core biopsy is more appropriate. The needle core biopsy can demonstrate a cellular fibroadenoma, hyalinised fibroadenoma or myxoid fibroadenoma. Needle core biopsies may assist in differentiating a fibroadenoma from a phyllodes tumour where possible. Histologically fibroadenomas have been classified as intracanalicular and pericanalicular, but this has no prognostic relevance and will not be referred to in this book.
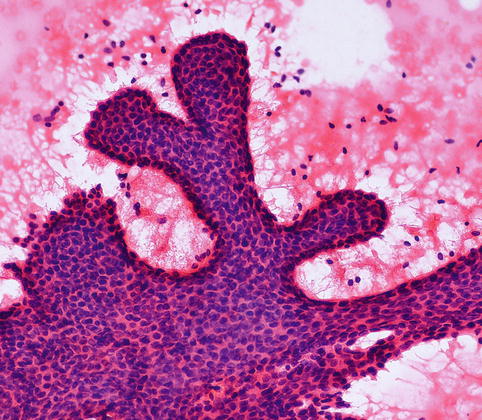

Fig. 8.7
FNAC from a fibroadenoma in an 18-year-old girl shows ‘staghorn’-shaped monolayer of cell with two cell types and bare nuclei in the background (H& E stain)
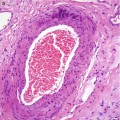
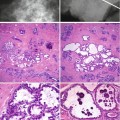
Macroscopically fibroadenomas exhibit a well-circumscribed nodule with a vaguely lobulated pattern, white firm cut surface with no haemorrhage or necrosis (Fig. 8.8). The histological features of a fibroadenoma are variable depending on the age of the patient. In a young patient below the age of 40, the fibroadenoma shows a mixture of epithelium separated by viable stroma (Fig. 8.9). There is no increase in cellularity of the stroma, cytological atypia or mitotic activity. Fibroadenomas in postmenopausal women, which are usually screen-detected, show hyalinised stroma and in this case there is associated calcification (Fig. 8.10). Myxoid fibroadenomas show loose stroma due to myxoid degeneration (Fig. 8.11). Myxoid degeneration can cause the fibroadenoma to increase in size during follow-up. Rarely myxoid fibroadenoma can be part of Carney’s syndrome. A complex fibroadenoma shows mixture of epithelial proliferation such as usual ductal epithelial hyperplasia, apocrine metaplasia and sclerosing adenosis, cysts with or without calcification (Fig. 8.12).
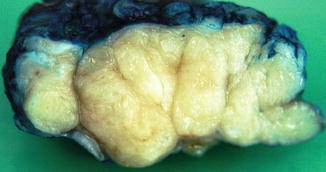
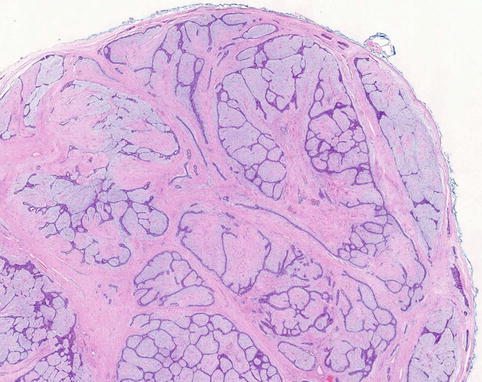
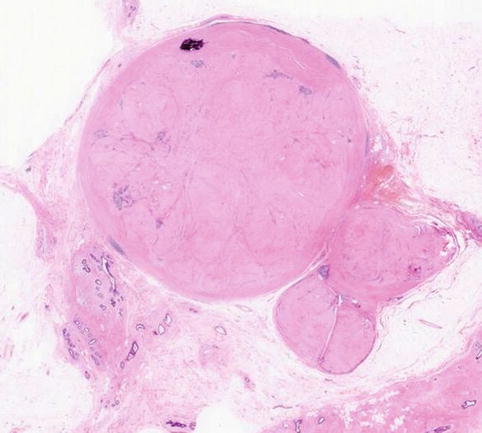
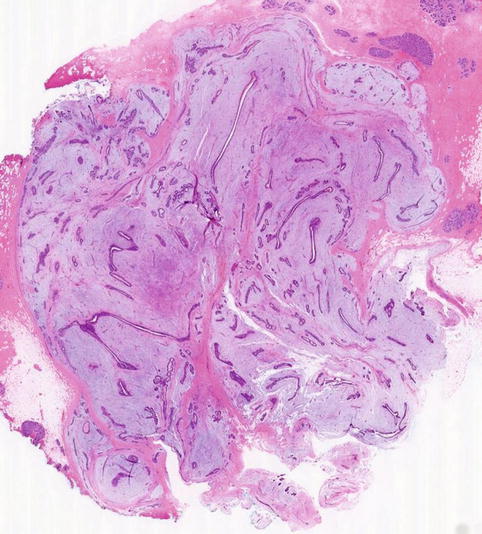
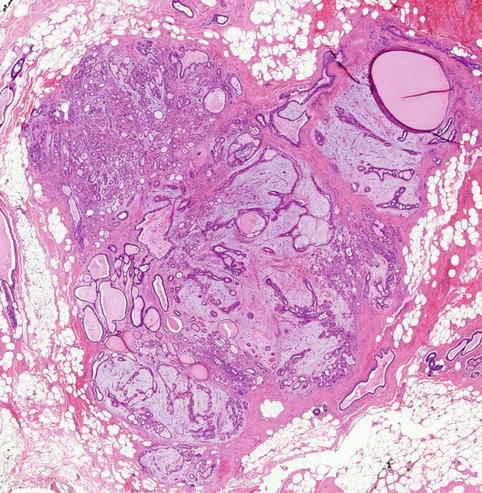

Fig. 8.8
Macroscopically a fibroadenoma has a white solid, vaguely lobulated appearance with no haemorrhage or necrosis

Fig. 8.9
Conventional fibroadenoma consisting mixed epithelial and fibrous proliferation to duplicate the macroscopic lobulated appearance. There is no increase in cellularity or cytological atypia

Fig. 8.10
Screen-detected fibroadenoma with a hyalinised stroma and focal calcification (From Fig. 8.2)

Fig. 8.11
Myxoid fibroadenoma consisting of loose stroma surrounding irregular epithelial proliferation. The border is ill-defined border. The ultrasound was reported as a heterogenous lobulated mass and graded as U3

Fig. 8.12
A complex fibroadenoma in a 54-year-old woman showing myxoid stroma, sclerosing adenosis and multiple cyst
8.1.6 Heterogenous Proliferations of a Fibroadenoma
Fibroadenomas can harbour an array of epithelial proliferations including mucin-filled ducts (Fig. 8.13) and columnar cell lesions (Fig. 8.14). Kuijper and colleagues (2001) carried out a systematic examination of 396 fibroadenomas from 358 patients to determine the histological features of epithelium and stroma within and adjacent to the fibroadenomas. The mean age of the patients was 33.4 ± 12.1 years (range 12–81 years). The sizes of fibroadenomas ranged from 0.1 to 22 cm, mean 1.5 ± 1.4 cm (SD).
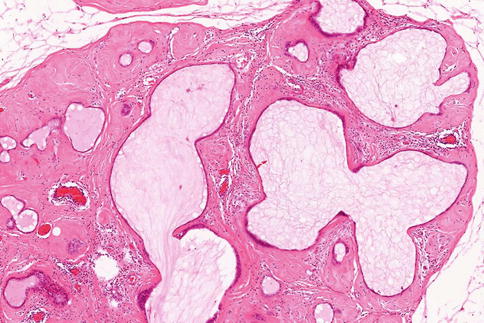
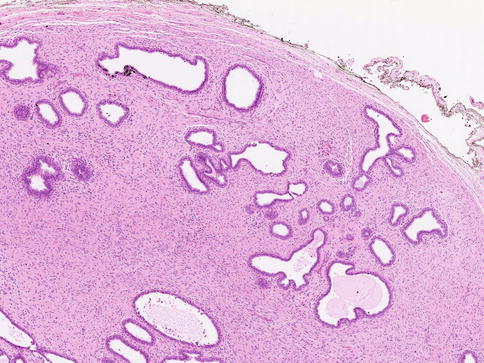

Fig. 8.13
A fibroadenoma containing multiple mucin-filled ducts or cysts in a hyalinised fibrous stroma

Fig. 8.14
A fibroadenoma containing columnar cell change characterised by distorted TDLU lined by columnar epithelium with apical snouts
Multiple fibroadenomas were identified in 28 patients (78 %), which were ipsilateral in 17 (61 %) patients and bilateral in 11 (39 %) patients. The main epithelial proliferations which the authors identified included mild ductal hyperplasia, 45 (11.6 %); moderate ductal hyperplasia, 106 (26.8 %); florid ductal hyperplasia, 21 (5.3 %); ADH, 1 (0.3 %) but no ALH; DCIS, 5 (1.3 %); LCIS, 3 (0.8 %) but no invasive carcinoma; fibrocystic change with apocrine metaplasia 111, (28 %); cysts, 20 (5.1 %); sclerosing adenosis, 49 (12.4 %); calcifications, 15 (3.8 %); microglandular adenosis, 1 (0.3 %); and papilloma, 7 (1.8 %). Other minor features identified were pseudolactational changes, squamous metaplasia, stromal pseudoangiomatous changes, stromal smooth muscle changes, foci of tubular adenoma and focal phyllodes tumour. Overall 4.39 % of the fibroadenomas contained epithelial hyperplasia, and when mild hyperplasia was excluded, significant hyperplasia was present in 32.3 % of the fibroadenomas. The complexity of the fibroadenoma was associated with the presence of epithelial hyperplasia. The mean age of the patients with DCIS and LCIS was 51.7 years which was significantly older than patients without the in situ carcinoma (CIS) (p < 0.001). CIS arising within a fibroadenoma and also present in the adjacent breast tissue was identified in three out of eight patients. 40.4 % of the fibroadenoma had complex features with 18 % of the fibroadenomas containing more than one complex feature, i.e. fibrocystic change with apocrine metaplasia, cyst, sclerosing adenosis and calcification. Complex fibroadenomas were seen more in older women (mean age: 35.4 years; p = 0.009).
Kuijper and colleagues also evaluated the proliferation activity in the tissue surrounding the fibroadenoma and reported the following features: mild hyperplasia, 16 (5.1 %); moderate ductal hyperplasia, 22 (6.9 %); florid ductal hyperplasia, 3 (0.9 %); ADH, 2 (0.6 %); ALH, 2 (0.6 %); LCIS, 1 (0.3 %); DCIS, 6 (1.9 %); invasive carcinoma, 3 (0.9 %); fibrocystic change with apocrine metaplasia, 75 (23.7 %); cysts, 8 (2.5 %); sclerosing adenosis, 46 (14.5 %); calcifications, 11 (3.5 %); microglandular adenosis, 6 (1.9 %); papilloma, 1 (0.3 %) and pseudolactational changes, 4 (1.3 %). The authors attributed the CIS arising within the fibroadenoma to be related to the high percentage of fibroadenomas with complex proliferations in their series and they concluded this increased the risk of subsequent carcinoma. However, they considered the presence of CIS and invasive carcinoma in the adjacent epithelium as coincidental. There was overall 8.8 % of fibroadenomas with hyperplasia in the surrounding tissue. The authors did not feel this was sufficient to calculate risk of cancer conferred by this external proliferation. In a separate study, Dupont et al. (1994) reported 13.7 % occurrence of hyperplasia in the adjacent tissue with an associated RR of cancer of 3.9. Most fibroadenomas were enucleated or excised with minimal surrounding tissue because they are benign lesions. Based on the findings of their study, Kuijper and colleagues advocate excision of fibroadenoma in women older than 35 years as this will remove potentially malignant lesions arising in fibroadenomas.
Sklair-Levy et al. (2008) reviewed 401 fibroadenomas and identified 63 (15.7 %) lesions which qualified as complex fibroadenomas. The average size of complex fibroadenomas was 1.3 ± 0.57 cm (range 0.5–2.6 cm) which was half the size of a simple fibroadenoma, average 2.5 ± 1.44 cm (range 0.5–7.5 cm), p < 0.001. On average, patients with complex lesions were 18.5 years older (median, 47 years; range, 21–69 years) than patients with simple fibroadenomas (median, 28.5 years; range 12–86 years), p < 0.001. Fifty-six (89 %) of complex fibroadenomas were graded as BI-RAD 3 or 4 on mammography or ultrasound and seven patients (11 %) had no imaging investigations. The diagnosis of complex fibroadenoma was made on needle core biopsy in 23 women (36.5 %), on needle core biopsy and excisional biopsy in 20 women (31.7 %), and on excisional biopsy alone in 20 women (31.7 %). Twenty-one of the 23 women with the diagnosis of complex fibroadenoma on needle core biopsy had no excisional biopsy and followed up; the masses were unchanged on mammography and ultrasound with a mean follow-up of 24.1 months (range, 10–48 months). The other two patients were lost to follow-up. Twenty patients had excisional biopsies following diagnosis of complex fibroadenoma on needle core biopsies; one patient had a diagnosis of invasive lobular carcinoma and two had phyllodes tumours. Based on these findings, the authors recommended that complex fibroadenomas are associated with a low risk of malignancy and should be managed as simple fibroadenomas if diagnosed on needle core biopsies. If there is no atypia, the lesions should be monitored on mammography and sonography biannually for 2 years and annually thereafter. This view is contradictory to Kuijper et al. 9999who attributed the presences of CIS in fibroadenomas to the presence of complex features in the fibroadenomas.
8.1.7 Is a Fibroadenoma a Hyperplastic or Neoplastic Lesion?
There is conflicting information as to whether a fibroadenoma is neoplastic or hyperplastic proliferation. Morphologically some authorities classify a fibroadenoma as hyperplastic because it is an abnormal development of lobule (Dixon 1999), and this is supported by the following factors (Hughes et al. 1987):
(a)
Use of special stains shows that each fibroadenoma develops from a single lobule.
(b)
Fibroadenomas histologically closely resemble hyperplastic lobules which are common in normal breasts.
(c)
Growth of most fibroadenomas stops after they reach 2–3 cm in diameter or a significant percentage of them spontaneously regress.
(d)
Fibroadenomas show some hormonal dependency as the normal breast, i.e. some fibroadenomas grow rapidly during pregnancy and they lactate.
Molecular pathology has not been helpful because there are publications which report a fibroadenoma as polyclonal lesion and thus hyperplastic and others as monoclonal and therefore neoplastic. Noguchi et al. (1993) assessed the clonality of 10 fibroadenomas and five phyllodes tumours using PCR to assess the Big chromosome-linked gene PGK (phosphoglycerokinase). The stromal and epithelial elements in the fibroadenomas were polyclonal, whereas the stromal elements in phyllodes tumour were monoclonal but the epithelium was polyclonal. In a separate study Kuijper et al. (2002) also confirmed the polyclonal nature of the fibroepithelial elements in a fibroadenoma and the stroma in phyllodes tumours was monoclonal. In contrast, Cavall et al. (2001) claimed alterations in 10 cell cultures from 10 fibroadenomas in chromosomes X, 12, 14, 20 and 22, suggesting a neoplastic phenotype.
8.1.8 Fibroadenoma as Risk Factor for Malignancy
Although there are several publications, mostly as case reports (Buzanowski-Konakry et al. 1975; Pick and Iossifides 1984; Umemura et al. 1994; Tissier et al. 2000) documenting the presence of in situ or invasive cancer arising in a fibroadenoma, there is no consensus as to whether the association is coincidental in older women at risk of cancer or whether the fibroadenoma is precancerous. Fibroadenomas arise from terminal duct–lobular units, the same functional unit for lobular and ductal carcinoma. This was thought to account for the increased risk of carcinomas in fibroadenomas in the older women. Pick and Iossifides (1984) reported the presence of lobular carcinoma in situ in 65 % of the 62 cases of carcinoma arising in fibroadenoma. These authors concluded that the presence of carcinoma in the fibroadenomas was coincidental rather than the fibroadenoma being a risk factor for the carcinomas. Other studies also reported a disproportionately high prevalence of carcinoma in situ (mostly lobular) arising in fibroadenomas (Buzanowski-Konakry et al. 1975; Ozzello and Gump 1985). The authors advocated that carcinoma arising in fibroadenomas should not be treated differently from that arising de novo.
Dupont and colleagues (1994) reviewed 2,458 fibroadenomas diagnosed between 1950 and 1968, and 558 (22.1 %) were classified as complex fibroadenomas. Lesions were classified as complex fibroadenomas if they contained cysts greater than 3 mm in diameter, sclerosing adenosis, epithelial calcification or papillary apocrine changes (Dupont and Page 1985




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree