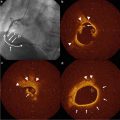
Fig. 9.1
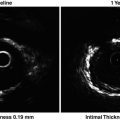
Endarterectomized carotid plaques show temperature heterogeneity
Human Ex Vivo Thermography Studies
The hypothesis that plaque temperature is a marker of local inflammation was originally proposed on the basis of observations from human ex vivo carotid endarterectomy specimens. Casscells et al. measured the intimal surface temperature of 20 sites of 50 samples of carotid plaques taken during surgical endarterectomy, using a sensitive needle thermistor. The measurements revealed several regions in which the surface temperatures varied from 0.2 to 0.3 °C, but 37 % of plaques had points not distinguished by naked eye, with substantially different temperatures (0.4–2.2 °C). These results were reproducible, while thermal heterogeneity (ΔΤ) could also be confirmed using an infrared camera in vivo. Furthermore, plaque temperature was directly correlated with inflammatory cell density (r = 0.68, p = 0.0001) and inversely proportional to the distance of the cell clusters from the luminal surface (r = −0.38, p = 0.0006). This was the first study demonstrating that localized heat is generated from inflamed atherosclerotic plaques in humans [15].
These observations were also confirmed in preliminary clinical studies. A correlation between the serum matrix metalloproteinase −1, −3, and −9 concentration with temperature difference in samples obtained through direct coronary atherectomy in eight patients has been reported [16]. In another study, in order to assess the possible contribution of infections to generation of heat in atherosclerotic plaques, the genus-specific monoclonal antibody CF-2 against Chlamydia pneumonia was used. Incubation of hot plaques with indomethacin showed a gradual decrease in plaque heat production over 5 h, suggesting an inflammatory origin of heat production in atherosclerotic plaques. However, no significant association between ΔΤ and Chlamydia pneumonia was found [17].
In pH readings in VP of human carotid endarterectomy specimens and atherosclerotic rabbit aortas, lower pH was associated with a higher temperature (r = 0.7; p < 0.01). Lipid-rich areas had a lower pH and a higher temperature, whereas calcified areas showed a higher pH and a lower temperature. Temperature and pH were significantly inversely correlated (r = 0.94; p < 0.01). This finding was in accordance with the assumption that lipid-rich vulnerable areas may have a more acidic environment [18].
Animal IVT Studies
Intravascular Thermography has been applied by several investigators in the past for the assessment of ΔΤ in animal models, validating the feasibility of this method in vivo. Naghavi et al. developed a contact-based “thermobasket” catheter for measuring in vivo the temperature at several points on the vessel wall in the presence of blood flow [19]. This catheter is equipped with four small, flexible wires with built-in thermocouples and a thermal sensor in its central wire for simultaneous monitoring of the blood temperature. The technical characteristics of the device are thermal resolution of 0.02 °C, thermal accuracy of 0.02 °C with a sampling rate of 20 temperature readings per second, and 7 sensors. The system was applied in a canine and a rabbit model of atherosclerosis. This catheter could detect ΔΤ over the atherosclerotic plaques in the femoral arteries of inbred atherosclerotic dogs and the aortas of Watanabe rabbits. In inbred cholesterol-fed dogs with femoral atherosclerosis, marked ΔΤ was measured on the atherosclerotic regions but not on disease-free regions (p < 0.05) (Fig. 9.2). Marked ΔΤ was also observed in the aortas of atherosclerotic rabbits but not in normal ones. The specific catheter showed satisfactory accuracy, reproducibility, and safety.
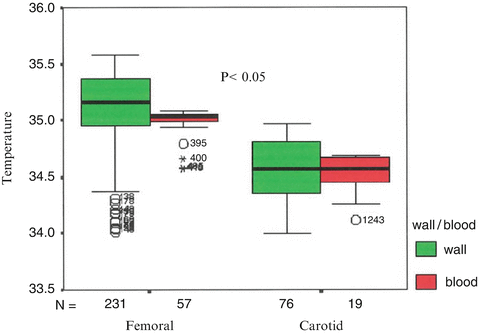

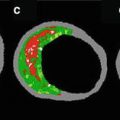
Fig. 9.2
The thermography basket catheter showed higher absolute temperatures as well as temperature heterogeneity on atherosclerotic lesions compared to lesion-free segments
Another over-the-wire thermography catheter with 4 thermistors was used in thermographic assessment of 20 rabbits: 10 in a normal diet and 10 after 6 months of cholesterol-rich diet. Marked ΔΤ (up to 1 °C) was detected in hypercholesterolemic rabbits at sites of thick plaques, as assessed by intravascular ultrasound (IVUS). In these same sites, histology showed a high macrophage density, but ΔΤ was absent at sites of plaques with a low macrophage density. The temperature heterogeneity detected in hypercholesterolemic rabbits was reduced significantly after 3 months of cholesterol lowering, while plaque histology showed a marked loss of macrophages but lack of changes in the plaque thickness [20]. In another study where rabbit atheromatic model was used, in vivo temperature measurements showed increased ΔΤ in plaques that contained higher density of macrophages, less smooth muscle cell concentration, and higher metalloproteinase-9 activity [21].
Human In Vivo IVT Studies
Many different types of intracoronary thermography catheters have been designed and important pathophysiological insights in the development of the unstable plaque have been obtained (Fig. 9.3). Intravascular Thermography had promising results as a method of assessment of plaque vulnerabilities, especially in specific group of patients, as well as in risk stratification and in evaluation of treatment (Table 9.1).
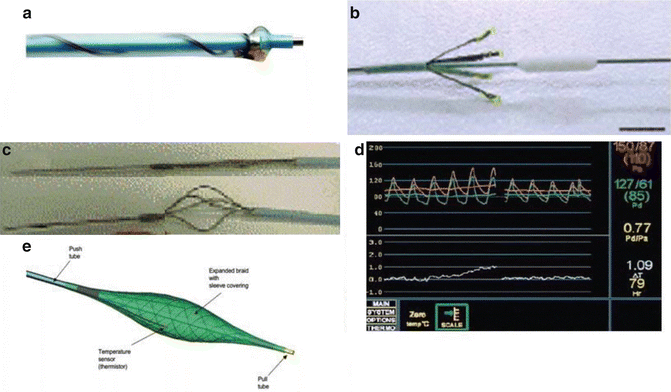

Fig. 9.3
(a) Epiphany thermography system (Epiphany, Medispes S.W., Zug, Switzerland). A monorail system containing two lumens with a temperature accuracy of 0.05 °C and a time constant of 300 ms. (b) Thermocore thermography system with a functional probe containing four thermistors with an accuracy of 0.01 °C. Epiphany coronary thermography system. (c) Volcano non-occluding thermography catheter (Volcano Therapeutics, Orange County, CA) with a self-expanding basket, five nitinol arms at its tip, with one thermocouple on each arm and another one on the central wire, allowing for real-time, cross-sectional thermal mapping of the arterial wall. (d) Radi PressureWire® (Radi Medical Systems, Inc., Uppsala, Sweden): a 0.014-in. wire that contains a high-sensitivity thermistor of 0.1 °C. (e) Accumed Systems, Inc. (Ann Arbor, MI, USA): A blood-flow-occluding feature with a temperature sensing structure at its distal end and a proximal end including a manually operated expansion control
Table 9.1
Human in vivo thermography studies
Author | Year | Main finding | Type of catheter |
|---|---|---|---|
Stefanadis et al. [22] | 1999 | Temperature differences between atherosclerotic plaque and healthy vessel wall increased through the clinical spectrum | Epiphany catheter: single-channel, thermistor-based |
Stefanadis et al. [31] | 2000 | Positive correlation of C-reactive protein and serum amyloid A with the temperature difference of the plaque | Epiphany catheter: single-channel, thermistor-based |
Stefanadis et al. [28] | 2001 | Increased local temperature in atherosclerotic plaques is strong predictor of an unfavorable clinical outcome in patients with coronary artery disease undergoing percutaneous interventions | Epiphany catheter: single-channel, thermistor-based |
Stefanadis et al. [30] | 2002 | Statin intake showed a favorable effect on heat release from atherosclerotic plaques | Epiphany catheter: single-channel, thermistor-based |
Webster et al. [42] | 2002 | Increased thermal heterogeneity detected in atherosclerotic plaques. No correlation with C-reactive protein was found | RADI PressureWire high-sensitivity thermistor |
Stefanadis et al. [35] | 2003 | Thermal heterogeneity was underestimated in atherosclerotic plaques due to the “cooling effect” of coronary blood flow | Epiphany catheter: single-channel, thermistor-based |
Stefanadis et al. [39] | 2003 | In vivo atherosclerotic plaque temperature recording was feasible with the new balloon-thermography catheter. Higher temperature difference was found after complete interruption of blood flow by inflation of the balloon | A balloon-thermography catheter designed for temperature measurements during coronary flow interruption. The thermistor probe is positioned at the distal segment of the catheter with a balloon at the opposite site. By inflation of the balloon, coronary flow is interrupted |
Schmermund et al. [24] | 2003 | Increased thermal heterogeneity in atherosclerotic plaques of patients with stable or unstable angina | Volcano catheter: self-expanding basket with five nitinol arms, one on each arm and one on the central wire |
Stefanadis et al. [43] | 2004 | Coronary sinus temperature was increased in patients with coronary artery disease and was found to be a prognostic factor for mid-term clinical outcome | A 7 Fr thermographic catheter possessing a steering arm at the proximal part of the catheter and a thermistor probe at the catheter tip. Manipulation of the steering arm proximally enables the distal end of the catheter to be curved (0–180°) |
Toutouzas et al. [23] | 2004 | Increased plaque temperature was observed for an extended period after myocardial infarction. Statins intake showed a beneficial effect after myocardial infarction on plaque temperature | Epiphany catheter: single-channel, thermistor-based |
Dudek et al. [44] | 2005 | Thermography was unable to differentiate between lesions at risk, despite a selection of lesions that should appear most distinct to differentiate | Volcano catheter |
Toutouzas et al. [45] | 2005 | Systemic inflammation correlated with coronary sinus temperature independently of the extent of coronary artery disease | A 7 Fr thermographic catheter possessing a steering arm at the proximal part of the catheter and a thermistor probe at the catheter tip. Manipulation of the steering arm proximally enables the distal end of the catheter to be curved (0–180°). |
Toutouzas et al. [27] | 2005 | Patients with diabetes mellitus had increased temperature difference compared to patients without. Statin intake showed beneficial effect on plaque temperature | Epiphany catheter: single-channel, thermistor-based |
Toutouzas et al. [46] | 2006 | Heat was generated in non-culprit lesions progressively increasing from patients with acute coronary syndrome to patients with stable angina | Epiphany catheter: single-channel, thermistor-based |
Rzeszutko et al. [47] | 2006 | Intracoronary thermography was safe and feasible. No ability to differentiate between lesions at risk, despite a selection of lesions that should appear most distinct to differentiate | Volcano catheter |
Worthley et al. [38] | 2006 | No significant temperature increase in patients with acute coronary syndrome, compared to baseline temperature | RADI PressureWire high-sensitivity thermistor |
Wainstein et al. [33] | 2007 | Intracoronary thermography detected vulnerable plaques, as these were assessed by intravascular ultrasound and atherectomy tissue histology | ThermoCoil guidewire |
Toutouzas et al. [34] | 2007 | Local inflammatory activation in non-culprit lesions correlated with systemic inflammation. Statins showed a beneficial effect on non-culprit lesion heat production | Epiphany catheter: single-channel, thermistor-based |
Toutouzas et al. [34] | 2007 | Culprit lesions with plaque rupture and positive arterial remodeling had increased thermal heterogeneity | Epiphany catheter: single-channel, thermistor-based |
Takumi et al. [48] | 2007 | Thermography showed accurate identification of the culprit lesion in patients with acute myocardial infarction and coronary total occlusion | RADI PressureWire high-sensitivity thermistor |
Cuisset et al. [32] | 2009 | Temperature increase across the lesion correlated with the pressure drop across the stenosis (R = 0.72, p < 0.001) | RADI PressureWire high-sensitivity thermistor |
Spectrum of ACS: First Clinical Study
The first clinical study with in vivo IVT performed by Stefanadis et al. in 1999 included 90 patients. The intracoronary thermography catheter utilized uses a thermistor-based sensor that contains two lumens (Epiphany; Medispes S. W., Zug, Switzerland). The first one runs through the distal 20 cm of the device and is used for the insertion of a guidewire (0.014-in.) that serves as a monorail provision system. The thermistor is positioned at the distal part of the thermography catheter of the second lumen. This catheter is 3, 3.5, or 4 F in diameter, depending on the size of the vessel, while the technical characteristics of this particular polyamide thermistor are: (1) temperature accuracy of 0.05 °C; (2) time constant of 300 ms; (3) spatial resolution of 0.5 mm; and (4) linear correlation of resistance vs. temperature over the range of 33–43 °C. The ΔΤ between atherosclerotic plaques and adjacent healthy segments increased progressively from control subjects through the ACS spectrum, from stable angina to acute myocardial infarction patients. Plaque ΔΤ was present in 20 %, 40 %, and 67 % of the patients with stable angina, unstable angina, and acute myocardial infarction, respectively, and did not correlate with the degree of stenosis. Temperature was constant within the arteries of the control subjects, whereas most atherosclerotic plaques showed higher temperature difference compared with healthy vessel wall [22]. Moreover, in another study including 55 patients, increased plaque temperature was observed for an extended period after myocardial infarction, indicating that the inflammatory process is sustained after plaque rupture. In patients with recent myocardial infarction ΔΤ was 0.19 ± 0.18 °C, while in patients with stable angina ΔΤ was 0.10 ± 0.08 °C (p = 0.03) [23]. Scmermund et al. observed ΔΤ in 50 % of patients with unstable angina and in 27 % of patients with stable angina. The range of difference was 0.14–0.36 °C. Although this study showed a difference between the two groups, there was still a considerable overlap [24].
IVT in Patients with Diabetes Mellitus
Inflammation of atherosclerotic lesions seems to be even more significant in diabetic patients. In a study including 45 patients with diabetes mellitus and 63 patients without, patients with diabetes mellitus had increased temperature difference compared to the control group. Patients with diabetes mellitus suffering from coronary artery disease showed increased local inflammatory involvement compared to patients without diabetes mellitus. This finding is in accordance with previous observations that patients with diabetes mellitus have more severe inflammation in their coronary atherosclerotic plaques, suggesting that diabetes mellitus has a strong impact on plaque destabilization via inflammatory activation. The inflammatory activation in patients with diabetes mellitus could be the explanation for the lack of favorable outcomes of trials in cardiovascular events, even in patients with intensive treatment to control blood glucose levels. Thus, the strict control of glucose levels with stabilization of local inflammatory involvement could potentially reduce the cardiovascular mortality in this high-risk group [25–27].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree