Fig. 1
Overview of the information collected from the subjects in SCAPIS. MRI magnetic resonance imaging, CT computed tomography, CCTA coronary computed tomography angiography, ECG electrocardiogram, HbA1c glycated hemoglobin, hsCRP high-sensitivity C-reactive protein (Adapted from Bergström et al. (2015).)
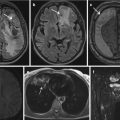
Imaging in SCAPIS focuses on cardiac, vascular, pulmonary, and metabolic profiling. Computed tomography (CT) is used to detect fat deposits in and around different organs. Multidetector CT (MDCT) of the lungs is used to detect and stage early signs of pulmonary disease, including emphysema and airway wall thickening. Atherosclerosis in the carotid arteries is assessed by ultrasound and, in participants with moderate-to-large plaques, magnetic resonance imaging (MRI). Coronary CT angiography (CCTA) is performed to detect plaques in the coronary arteries.
SCAPIS aims to combine these advanced imaging techniques with large-scale genotyping and recent developments in metabolomics and proteomics to build a unique data, blood and image bank, which will significantly contribute to improvements in the prediction of prognosis, prevention, and treatment of MI, stroke, and COPD. However, an inevitable consequence of exposing a large number of subjects to an extensive imaging protocol is that a considerable number of both expected and unexpected clinical findings will be identified. One of the main aims of the pilot study was to assess the volume and type of clinical findings that would arise and to develop a clinical workflow to handle them. Emphasis was put on addressing the ethical issues that inevitably would arise in either informing or choosing not to inform the subjects. Early on, the study organization decided that we had an ethical obligation to inform the subjects of all clinical findings that at the time of the examination were of relevance for the present or future health of a subject. In contrast, participants will not be individually informed on results arising as a result of future research.
Evaluation of the pilot study confirmed that a substantial number of clinical findings were identified in the clinically mostly healthy volunteers. Here we focus on our handling of pulmonary nodules and asymptomatic coronary artery stenosis found after imaging with CT – clinical features that were observed in a large number of participants and that posed great challenges for SCAPIS in their practical and ethical handling.
2 Computed Tomography Imaging
The following protocols were used for pulmonary and cardiac imaging in SCAPIS. The protocols can be found and were originally published as a supplemental file in Bergström et al. 2015. All computed tomography (CT) scanning is performed on a Somatom Definition Flash (Siemens Healthcare, Forchheim, West Germany) with a stellar detector. Care Dose 4D, Care kV, and SAFIRE are used for dose optimization in some protocols. All sites are equipped with similar scanners and, in agreement with the vendor, no software or hardware updates are allowed during the study period.
2.1 Preparation of Subjects
Before undergoing a scan, subjects only eat light meals and avoid beverages containing stimulants (e.g., caffeine). Two hours before a scan, the participants are given a standardized meal (Modifast, Nutriton & Santé) calculated based on body mass index to achieve a stable metabolic state.
2.2 CT Examination
To plan the examination, topograms are performed in a lateral view of the thorax (scan length 512 mm) and an anterior-posterior view starting at the chin ending below the knee (scan length 1536 mm). For scan parameter details and protocol parameters, see Bergström et al. 2015.
2.2.1 Lung Images
Lung images are acquired using spiral scanning.
2.2.2 Cardiac Imaging: Coronary Artery Calcium Score (CACS) and Coronary CT Angiography (CCTA)
All cardiac imaging is ECG triggered. Renal function is assessed and potential contraindications identified to exclude subjects for whom contrast media administration could pose a risk.
Unless contraindicated, a β-blocker (metoprolol) is administered to the subjects to reduce heart rate to below 60 beats/min without too much reduction in blood pressure. This is done either 1.5 h before the scan by oral administration of 25–50 mg metoprolol and/or directly at the scan using intravenous administration of 2.5–15.0 mg metoprolol dependent on heart rate and blood pressure. In addition, subjects with a systolic blood pressure >110 mmHg are given two doses of sublingual glyceryl nitrate (4 mg/dose, GmbH & Co, KG).
For CCTA, the contrast media iohexol is administered (Omnipaque, GE Healthcare, 350 mgI/mL). The individual dose is 325 mg I/kg body weight, and the injection time is 12 s. To plan the contrast media delay time for the CCTA, a sequential scan covering 10 mm is performed using a test bolus of 10 ml contrast media (scan delay 8 s).
Two different CACS protocols are available. A flash spiral protocol is used for subjects with a body weight ≤90 kg and a regular heart rate. For all others, a sequential protocol is used. Five different CCTA protocols are used; the choice is determined according to heart rate, the regularity of the heart rate, and body weight. CCTA 1 is chosen if there are no calcifications on CACS, the subject has a regular heart rate ≤60 beats/min and a body weight ≤85 kg. CCTA 2 is chosen if heart rate is relatively stable (≤75 beats/min) and if the CT system can deliver a sufficient radiation dose for desired image quality. This is mainly related to the size of the subject. CCTA 3 is chosen if heart rate is relatively stable (>75 beats/min), and the CT system can deliver a sufficient radiation dose for the desired image quality. CCTA 4a and 4b are chosen when the heart rate is relatively stable, but radiation dose in relation to the size of the subject needs to be increased to reach an optimal image quality. It is done by using either a sequential technique with increased rotation time (0.33 s) or a spiral technique using both x-ray tubes. For the spiral technique, the pitch is chosen according to heart rate. CCTA 5 is chosen if the subject has an irregular heartbeat (e.g., atrial fibrillation or if the electrocardiogram indicates a variance of heart rate exceeding 4 beats/min). The protocol uses a spiral technique, and the pitch is chosen according to heart rate.
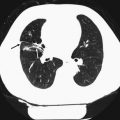
3 Pulmonary Nodules
3.1 Guidelines for Follow-Up of Pulmonary Nodules
A pulmonary nodule identified by CT is defined as a rounded or irregular opacity that measures up to 3 cm in diameter and that may be solid, part-solid, or non-solid (also known as a ground-glass nodule) (Hansell et al. 2008). Before the widespread use of CT, the accepted standard of care was to regard all non-calcified pulmonary nodules as potentially malignant lesions until proven stable over a period of 2 years (Tan et al. 2003). With increasing evidence in the scientific literature indicating that very few small nodules (<4–5 mm) would prove to be malignant over a 2-year period, the Fleischner Society proposed new guidelines in 2005 (MacMahon et al. 2005). These recommendations state: no follow-up for low-risk patients with nodules ≤4 mm; 1–3 follow-up CT scans for up to 2 years (depending on nodule size and patient risk factors) for solid nodules measuring 4–8 mm (longer follow-up time for ground-glass and part-solid lesions); and that further investigations such as positron emission tomography, percutaneous needle biopsy, or thoracoscopic resection for nodules >8 mm could be considered (MacMahon et al. 2005). A recent addition to these guidelines stated that non-solid lesions >5 mm and part-solid lesions should have an initial follow-up with CT at 3 months, with yearly follow-up for up to 3 years if the nodule is unchanged or biopsy or resection if the solid part measures ≥5 mm at the 3-month follow-up (Naidich et al. 2013). There is currently no lung cancer screening program in Sweden, but recently published national guidelines (Cancercentrum 2015), published after the pilot SCAPIS adhere to the previously mentioned recommendations regarding the handling of incidentally detected pulmonary nodules (MacMahon et al. 2005; Naidich et al. 2013).
In addition to nodule size, morphological characteristics of the nodule should be evaluated. Pseudocavitation, air bronchograms, cavitation, spiculation, non-solid components, and punctate and eccentric calcification are considered as features of a malignant nodule (Edey and Hansell 2009). Central, diffuse, or laminated calcification suggests a benign lesion, and the combination of fat and calcification indicates a hamartoma. It is also important to recognize benign perifissural nodules to reduce the number of follow-up examinations required for the workup of suspicious nodules (de Hoop et al. 2012).
3.2 Handling of Incidental Pulmonary Nodules in the Pilot Trial
The recommended follow-up CT examinations aim at detecting nodule growth, which is a strong lung cancer predictor (Gould et al. 2013). Both the inclusion of nodules into follow-up schemes and the detection of nodule growth are highly dependent on measurement accuracy, where the variability of two-dimensional measurements and challenges in sometimes segmenting reliable 3D volumes are well-known problems (Nair et al. 2012). The detection rates of small pulmonary nodules are influenced by a number of factors. The reconstructed slice thickness of the CT scan is one of the most important technical parameters, and the use of maximum intensity projection (MIP), volume-rendered slabs, or computer-assisted detection improves nodule detection when compared with evaluation of standard axial images (Edey and Hansell 2009).
In the pilot SCAPIS, the radiologists evaluated the participant’s chest CT examinations by reviewing submillimeter as well as thicker MIP slices with the purpose of improving the nodule detection rate. Detected nodules were morphologically characterized, and two-dimensional measurements were performed at a standardized small display field of view to reduce measurement variability. Solid nodules measuring ≤4 mm were reported only in the electronic case reporting file (eCRF) because of the low anticipated risk of a malignant lesion. All participants with nodules ≥5 mm, where definite benign characteristics could not be established, were referred to the pulmonary medicine department. For participants with solid nodules measuring ≥10 mm, further workup was suggested, whereas participants with solid nodules measuring 5–9 mm had a recommendation of CT surveillance at 6, 12, and 24 months. The limit of 10 mm was chosen based on awareness of measurement variability and in accordance with the clinical routine in the west region of Sweden at the time of the study. Follow-up was also recommended for participants with part-solid and non-solid nodules measuring 5–10 mm.
In total, around 40 % of the 1111 participants in the pilot SCAPIS presented with pulmonary nodules, around 11 % of the population were referred to the pulmonary medicine department, and CT surveillance was recommended for around 10 %. The majority of the participants completed their follow-up CT examinations and, as expected, very few nodules exhibited detectable growth even when nodule volumes were measured.
3.3 Handling of Incidental Pulmonary Nodules in the Main Trial
The health-care resources required to follow-up the incidentally detected nodules in the pilot SCAPIS became a major concern and threatened the feasibility of the full-scale SCAPIS. In September 2013, the lung expert group of SCAPIS met with representatives from the NELSON trial and discussed at that timepoint unpublished data, which was later published by Horeweg et al. (2014). As a result of this meeting, SCAPIS decided to implement the following: A finding of a solid nodule with a volume <100 mm3 should only be reported in the eCRF. This decision was based on results from the NELSON trial showing that lung cancer probability did not significantly differ between participants who had nodules measuring <100 mm3 and those who had no detected nodules. Participants with a solid nodule measuring ≥300 mm3 should be referred to pulmonary medicine specialists for additional workup, as this finding has been shown to indicate a participant at high risk of developing lung cancer (Horeweg et al. 2014). Participants with solid nodules measuring 100–299 mm3 should be scheduled for a follow-up CT scan at 3 months, and smokers should have an additional follow-up CT scan at 24 months.
In the first year of SCAPIS at the Gothenburg site (n = 1719), the revised strategy compared with the pilot trial has reduced the number of participants referred to CT surveillance and direct clinical workup down to around 7 % and 2 %, respectively (Note: data on solid nodules only). It was also decided that the determination of nodule growth in the follow-up CT scans should be based on volumetric analysis and volume doubling time as suggested from the analysis of data from the NELSON trial (Horeweg et al. 2014).
Participants identified with non-solid and part-solid nodules are followed up in SCAPIS in accordance with the recently published Swedish national recommendation for such nodules: an initial follow-up with CT at 3 months for nodules ≥5 mm, with yearly follow-up CT surveillance for up to 36 months or clinical work-up depending on nodule characteristics (Cancercentrum 2015).
Recently, the cost-effectiveness of follow-up of incidentally detected pulmonary nodules on CT has been questioned (Goehler et al. 2014). Although nodule follow-up programs are expected to result in a small reduction in lung cancer mortality, the cost is substantial per quality-adjusted life-year, especially in nonsmokers (Goehler et al. 2014).
4 Asymptomatic Coronary Artery Stenosis
4.1 Guidelines for Follow-Up of Coronary Artery Stenosis
In the current European Society of Cardiology (ESC) guidelines, a patient with documented coronary artery disease (CAD) is categorized as having a >10 % risk for cardiovascular death within 10 years (Perk et al. 2012). Documented CAD is defined as a positive invasive or non-invasive test, e.g., invasive coronary angiography (ICA) or nuclear imaging, or previous CAD, peripheral artery disease, or ischemic stroke.
In SCAPIS, CCTA is used to investigate plaques in coronary arteries and will therefore uncover cases of obstructive CAD. However, the ESC guidelines do not explicitly refer to CCTA-identified CAD in a sample of randomly selected mostly healthy subjects and are therefore difficult to directly apply to the SCAPIS population. Furthermore, because CCTA has previously not been used in such a large cohort of mostly healthy, asymptomatic subjects randomly invited to an examination, there is almost a complete lack of information on prognosis and no valid guidelines exist on which treatment to recommend and who should be further evaluated.
Several studies have shown an association between CCTA-identified CAD, both non-obstructive and obstructive, and mortality (Ostrom et al. 2008; Hadamitzky et al. 2009; Abdulla et al. 2011; Chow et al. 2011). CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) recently published a follow-up of 7950 patients without chest pain and reported that the 2.5 year mortality rate was 4.1 % in patients with obstructive CAD and 1.7 % in patients without obstructive CAD (Cho et al. 2012). Patients with high-risk CAD (defined as left main stenosis >50 % or 3-vessel disease) have worse prognosis regardless of whether the patient is symptomatic or not (Chow et al. 2011; Cho et al. 2012).
4.2 Challenges with Coronary Calcifications
Plaques with large calcifications are a challenge to CCTA and tend to cause an artifact commonly called calcium blooming, which can result in overestimating the degree of obstruction caused by the plaque or in some cases prevent assessment of the plaque (Kroft et al. 2007; Dey et al. 2008). Calcium blooming is one explanation for why the positive predictive value of CCTA is low compared with invasive coronary angiography (ICA) (Husmann et al. 2008).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree