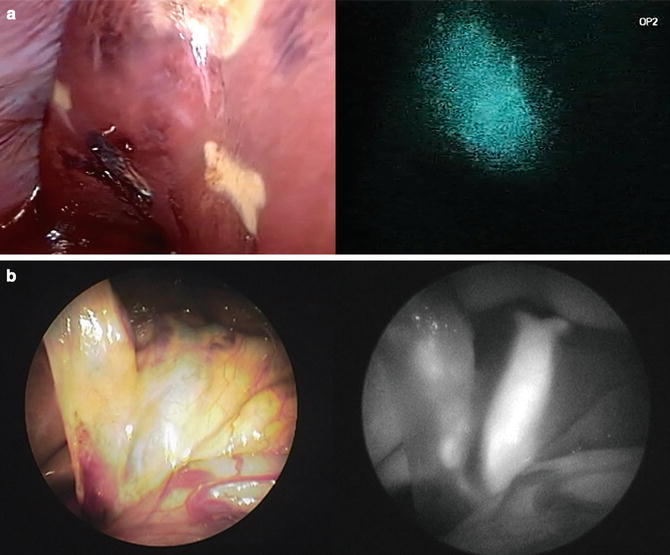
Fig. 7.1
PDE® system and its application to liver resection. (a) Camera and control units of PDE®. (b) Hepatocellular carcinoma (HCC) (arrow) is visualized on the liver surface by PDE®. (c) Colorectal liver metastasis (arrow) is identified on the liver surface prior to liver resection by pde-neo®, which can switch between color images and fluorescence images. (d) Prototype imaging system enables visualization of indocyanine green (ICG)-specific fluorescence emitted by an HCC as pseudo-color images (see Video 7.1)
The major advantage of PDE® lies in its size and portability, which enables the surgeon to hold the camera unit (covered with a sterilized drape) and easily obtain fluorescence images from various angles during surgery. With this system, the ICG fluorescence emitted from target organs is visualized on a background of monochromatic structures using autofluorescence of other organs within near-infrared range (Fig. 7.1b). The second clinically available model (pde-neo®) consists of a CCD camera for obtaining color images and enables switching to fluorescence images from color images (Fig. 7.1c). Currently, the next-generation model is under development to extract ICG fluorescence from autofluorescence, thereby showing ICG-specific fluorescence as pseudo-color images on a monochromatic background (Fig. 7.1d).
HyperEye Medical System
The HyperEye Medical System (Mizuho Ikakogyo, Tokyo, Japan) (Fig. 7.2a) was originally devised to identify coronary arteries during cardiovascular surgery [9]. It was first marketed in Japan in 2010. Since then, application of this system has been extended to sentinel node biopsy [10] and fluorescence cholangiography/angiography during digestive surgery and liver transplantation [11].
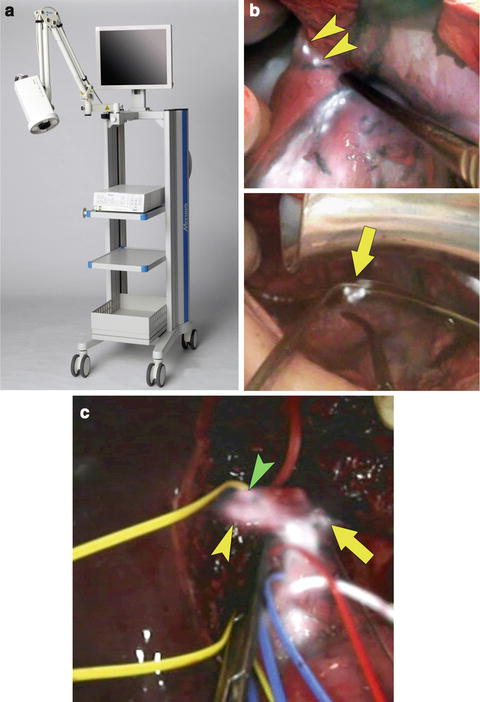

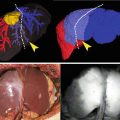
Fig. 7.2
HyperEye Medical System and its applications to hepatobiliary surgery. (a) Appearance of the HyperEye Medical System. (b) HyperEye Medical System enables visualization of fluorescing tiny nodules of HCC invading the diaphragm (arrowheads) on color images (top) during resection of disseminated nodules caused by percutaneous radiofrequency ablation. Simultaneous visualization of fluorescence images and color images helps surgeons detect disseminated nodules to be removed (arrow, bottom) (see Video 7.2). (c) Fluorescence cholangiography following intrabiliary injection of ICG solution (0.025 mg/mL) identifies the confluence between the left hepatic duct (arrow) and branches of the right hepatic ducts draining the right lateral sector (yellow arrowhead) and right paramedian sector (green arrowhead) during donor right hepatectomy (see Video 7.3)
This system enables ICG-fluorescence images to be superimposed on color images, which helps surgeons understand spatial relations between the target structures containing ICG and surrounding organs (Fig. 7.2b, c). In the original model, the camera imaging head with a 10× optical zoom was mounted on the imaging system to obtain ICG-fluorescence images from outside the surgical field without blurring of images caused by hand movement. A newer model, composed of a portable camera unit such as PDE®, is being developed for marketing in countries outside of Japan.
Prototypes of Laparoscopic Fluorescence Imaging System
In addition to commercially available laparoscopic fluorescence imaging systems described in the following chapters, several prototypes have been used in the clinical setting in Japan. In 2004, Nimura and colleagues reported clinical application of infrared ray electronic endoscopy (Olympus Optical, Tokyo, Japan) for detecting sentinel lymph nodes during gastric cancer surgery through absorption of infrared rays by ICG [12]. This technique led to the development of a laparoscopic imaging system visualizing ICG fluorescence under illumination by near-infrared light (Olympus Medical Systems, Tokyo, Japan) (Fig. 7.3a). Another prototype laparoscopic imaging system (Hamamatsu Photonics and Shinko Optical, Tokyo, Japan) (Fig. 7.3b) has been developed for sentinel node navigation surgery in gastrointestinal cancer [13] and evaluation of the placental vascular network in the treatment of twin–twin transfusion syndrome [14]. Although these standard-definition models already provide high-contrast, sensitive fluorescence images sufficient for real-time identification of the bile ducts [15], liver cancer [16], and hepatic segments [17] during laparoscopic surgery, the quality of both the color and fluorescence images should be further improved to meet the standard of high-definition imaging and display before marketing.