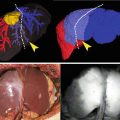
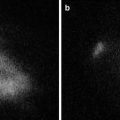
Fig. 32.1
A perforator flap
Various methods have been used to determine the anatomy of the blood-supplying vessels [2], according to the type of flap. For example, the preoperative perforator mapping of a fibular flap or anterolateral thigh flap is often made by Doppler sonography [3]. But this mapping requires an experimented operator who is not necessarily the operating surgeon. Furthermore, the images obtained with this technique are not very contributive. CT angiography has also been used to map anterolateral thigh flaps or deep inferior epigastric flaps [4]. But it is an irradiating method that requires the injection of potentially allergenic iodine contrast agent. Its sensibility has not been determined yet.
Indocyanine green fluorescence angiography (ICGFA) is used to explore superficial vascularization. It is particularly well adapted to evaluate skin perfusion. It should help to localize perforator vessels [5]. It has been also used for intraoperative assessment of flap perfusion and for postoperative flap monitoring [6]. We tested ICGFA in reconstructive surgery using the Fluobeam™ device (Fluoptics, Grenoble, France) for flap planning and flap monitoring (Fig. 32.2).
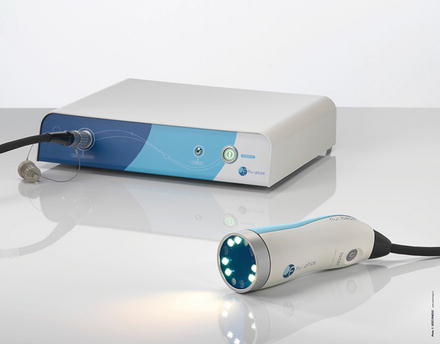

Fig. 32.2
The Fluobeam system for optical angiography
ICGFA for Flap Planning
The contribution of ICGFA for flap planning was assessed in animal studies. Giunta et al. correlated fluorescent perfusion index to flap necrosis in random pattern anterior abdominal rat flaps [7]. Lee et al. localized perforator vessels of epigastric flaps in pigs with near-infrared fluorescence imaging [8]. They found a 100 % correlation between the number of perforator vessels identified by fluorescence, x-ray angiography, and anatomic dissection. More recently, Onoda et al. compared perforator localization with computed tomography (MDCT), Doppler flowmetry, and ICGFA [9]. They concluded that “the identification of perforators was difficult with ICGFA in patients with a flap thickness >20 mm.”
We performed a cadaveric study to confirm the feasibility and accuracy of ICGFA for flap planning, before its clinical use in humans. We also had for objective to define the ergonomics of a fluorescent system dedicated to reconstructive surgery. We explored anterolateral thigh flap perforators on 22 cadavers. Femoral vessels were exposed just below the inguinal ligament and catheterized.
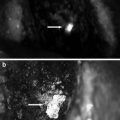
In a first step, 20 mg of Monopeak Indocyanine Green (Infracyanine™, SERB laboratory, Paris, France) was injected and washed out with 20 ml of saline. The Fluobeam™ probe (Fluobeam FB800, Fluoptics, Grenoble, France) was fixed on an articulated arm, 20 cm above the thigh skin, and recording was started at the time of saline injection (Fig. 32.3). Perforator vessels appeared as fluorescent spots after a few seconds (Fig. 32.4). Those spots were marked on the skin and localized in an orthogonal landmark defined by the line joining the iliac crest to the patella and the perpendicular to its middle. Femoral vessels were washed out again with saline at the end of the fluorescence examination.
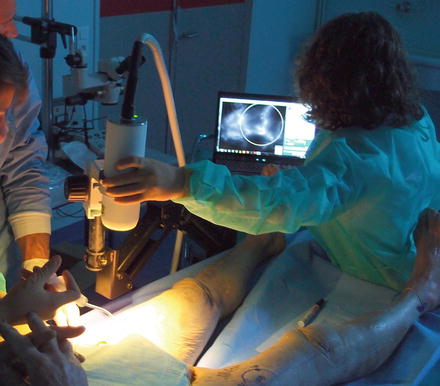
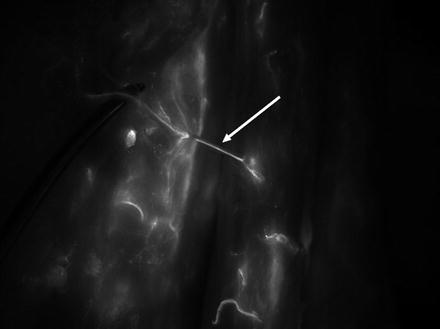

Fig. 32.3
Fluobeam™ system set-up to explore perforator vessels of the thigh

Fig. 32.4
Thigh. Perforator vessels appeared as fluorescent spots after a few seconds
In a second step, the thigh was then detached above the iliac crest and below the knee. All the visible vessels on the distal cut were ligatured (especially the popliteal vessels) to prevent leaking during the injection of 20 ml of iodine solution (Omnipaque, 350 mg I/ml) for computed tomography angiography (CTA). The perforators identified by CTA were localized in the same landmark after reconstruction.
The last step was dissection of the thigh to expose the perforator vessels and to localize them, always in the same landmark. The coordinates of perforators given by CTA and ICGFA were compared with the coordinates given by dissection (gold standard); 97 perforator vessels were localized by ICGFA, 11 by CTA, and 76 by dissection. Unlike CTA, ICGFA localization was correlated to dissection; it was able to reveal even very tiny perforators. But it specificity was poor (14.7 %). ICGFA showed all the skin vascularization, it did not differentiate between surgically useful perforators and other superficial vessels. Nevertheless, we concluded that ICGFA would help to plan perforator flaps, when combining its data with anatomical knowledge. This anatomical study also allowed validating the ergonomics to transfer this technology for clinical trials, and even in the operating room.
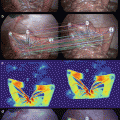
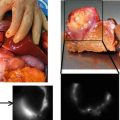
The goal of our clinical study was to validate the relevance of ICGFA for perforator flap planning, and to compare it with the gold standard (i.e. surgical dissection) and with routine investigation in our center. Twenty patients were included. Reconstruction was planned with one of the three following flaps: fibular flap, anterolateral thigh flap (ALT), or deep inferior epigastric flap (DIEP). Routine planning was made with echo-Doppler for fibular flaps and with CTA for ALT or DIEP in our department. The identified perforator vessels were localized in a specific landmark according to the flap (Fig. 32.5).
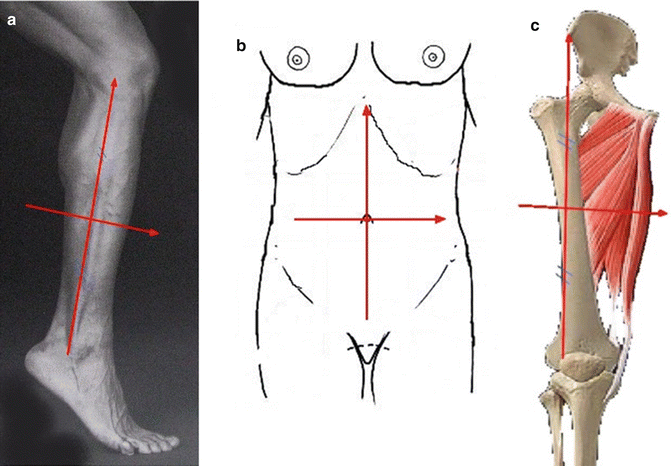

Fig. 32.5
The identified perforator vessels were localized in a specific landmark according to the flap
The day before surgery, 0.025 mg/kg of Infracyanine™ was injected intravenously and the skin area of the flap was explored with the Fluobeam™ system. Superficial vessels appeared as fluorescent spots and were marked with a pen on the skin and localized in the specific landmark (Video 32.1).
The position of perforator vessels was determined per-operatively in the same landmark after dissection. The three sets of localization coordinates (surgery, ICGFA, Doppler or CTA) were compared, surgical data being the reference.
ICGFA localization was accurate in a range of 0.90 cm ± 0.96, compared to anatomical localization. It was more accurate than echo-Doppler for fibular flaps (1.28 cm ± 1.01). But its specificity was poor (48 %); as in the cadaveric study, because it revealed all the superficial vascularization and not just the perforator vessels useful for surgery. Nevertheless, it was possible to differentiate and to identify the targeted vessels that first lit up in the area where they were supposed to be, according to surgical experience.
One of the difficulties with fluorescence angiography is the rapidity of skin illumination; the spots of the perforator vessels are very quickly drowned in the surrounding fluorescent noise, and it becomes hard to localize the perforator vessels accurately. A solution is to use a fluorescent ruler stitched on the skin, and to record the fluorescent data on video. The video can then be viewed image by image to improve optical localization (Video 32.2).
These data need to be confirmed with more patient studies. Nevertheless, ICGFA seems to be an accurate and safe method for perforator localization. It can be used directly by the surgeon, and does not require any irradiation or iodine contrast agent.
ICGFA for Flap Monitoring
The major complication of flap surgery is the obstruction of nutrient vessels (arterial and/or venous thrombosis). This can lead to flap failure (necrosis) with dramatic functional and esthetic consequences, if not treated rapidly. Flap monitoring is critical [10]. The most common technique for flap monitoring is clinical assessment. The surgeon monitors skin flap color, flap temperature, and capillary refill (ring test). In case of doubt, he can puncture the flap to verify bleeding, but this may harm the flap. This technique is very dependent on surgeon experience and signs of complication (thrombosis) appear late.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree