Human pancreatic cancer cell lines
In vitro
+
−
Mia Paca-2
x
FG
x
BxPC-3
x
CFPAC
x
Panc-1
x
Capan-1
x
XPA-1
x
XPA-3
x
XPA-4
x
In vivo
+
−
ASPC-1
x
BxPC-3
x
CFPAC
x
Panc-1
x
Capan-1
x
Human colon cancer cell lines
In vitro
+
−
LOVO
x
HCT-116
x
SW948
x
LS174T
x
HT-29
x
SW480
x
In vivo
+
−
LS174T
x
Colo4104
x
Immunofluorescence Staining of Tissue for Binding with Anti-CEA Antibody
Screening of normal human tissue samples for binding to conjugated anti-CEA antibody used immunofluorescence staining of a human tissue array. This array contained two samples each of 19 different noncancerous adult human tissues including: salivary gland, liver, small intestine, stomach, kidney, skeletal muscle, skin, heart, placenta, breast, cervix, uterus, spleen, lung, brain, thyroid, pancreas, ovary, and adrenal gland. Human tumor grown in nude mice from the pancreatic cancer cell line ASPC-1 and the primary human colon cancer tumor Colo4104 were used as positive controls, and mouse axillary lymph node tissue was included as a negative control. Both the ASPC-1 pancreatic tumor and the Colo4104 colon tumor yielded positive staining for CEA. In noncancerous tissues the majority of samples did not demonstrate binding of conjugated anti-CEA above the isotype-control IgG background. A low level of staining above background was present within the small intestine and cervix. Notably, the normal colon and pancreas did not bind conjugated anti-CEA. Table 22.2 denotes the staining for all noncancerous human lists samples tested [12].
Table 22.2
CEA expression in adult human tissues
Tissue | Staining |
|---|---|
Salivary gland | − |
Liver | − |
Small intestine | +/− |
Stomach | − |
Kidney | − |
Skeletal muscle | − |
Skin | − |
Heart | − |
Placenta | − |
Breast | − |
Cervix | + |
Uterus | − |
Spleen | − |
Lung | − |
Brain | − |
Thyroid | − |
Pancreas | − |
Ovary | − |
Adrenal gland | − |
ASPC-1 tumora | +++ |
Colo4104 tumora | ++ |
Mouse axillary LNb | − |
Fluorophore-Conjugated Anti-A Antibody for the Intraoperative Imaging and FGS of Pancreatic and Colorectal Cancer
In 2008, our team was the first to evaluate the use of a fluorophore-labeled anti-CEA monoclonal antibody to aid in primary and metastatic cancer visualization and FGS in nude mouse models of human colorectal and pancreatic cancer [12]. Anti-CEA conjugated with a green fluorophore, resulted in improved resection. Subcutaneous, orthotopic primary and metastatic human pancreatic and colorectal tumors were readily visualized with fluorescence imaging after administration of conjugated anti-CEA. The fluorescence signal was detectable 30 min after systemic antibody delivery and was stable for 2 weeks, with minimal in vivo photobleaching after exposure to standard operating room lighting. We demonstrated the principle that fluorophore-conjugated antibodies enabled successful FGS in orthotopic mouse models of pancreatic cancer [17].
Imaging of Subcutaneous Tumors with Fluorescent Anti-CEA Antibody
The human pancreatic cancer cell lines ASPC-1, BxPC-3, CFPAC, Panc-1, and Capan-1 were implanted subcutaneously in nude mice and evaluated for CEA expression. One colon cancer cell line (LS174T) and a primary human colon cancer patient specimen (Colo4104) , grown in nude mice, were also implanted subcutaneously. When the tumors had reached approximately 1–2 mm diameter, the animals were each given a single dose of Alexa Fluor 488-conjugated anti-CEA or IgG i.v. All 5 pancreatic cancer cell lines implanted bound the CEA as did the colon cancer cell line as well as the patient colon cancer model demostrated by fluorescence intensity above background IgG (Table 22.1).
Imaging Orthotopic Tumors with Fluorescent Anti-CEA Antibody
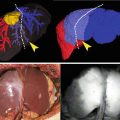
Tumors implanted orthotopically into the nude mouse pancreas and colon were evaluated for imaging using conjugated anti-CEA. The human pancreatic cancer cell lines ASPC-1 and BxPC-3 were used. For the colon cancer, the patient tumor Colo4014 was used in a model termed patient-derived orthotopic xenograft (PDOX®) [30, 31]. Orthotopic pancreatic or colon tumor-bearing mice were given a single dose of Alexa Fluor 488-conjugated anti-CEA or IgG via tail vein injection 7–10 days after tumor implantation. The animals were imaged under both bright field and fluorescence illumination using the Olympus variable-magnification OV100 Small Animal Imaging System [32]. Intravital fluorescence imaging revealed very small ASPC-1 and BxPC-3 tumors which were not visible under bright-field illumination, even at higher magnification (Fig. 22.1a–c). With fluorescence imaging it was clear that the extent of tumor invasion was much greater than that seen under standard bright light (Fig. 22.1d, e). The tumors in the colon cancer-bearing animals were visible under both bright field and fluorescence imaging at the magnification used (Fig. 22.2a–e). The animals which received control conjugated IgG had no green fluorescence in either their pancreatic (Fig. 22.1a, b) or colon (Fig. 22.2a, b) tumors [12].
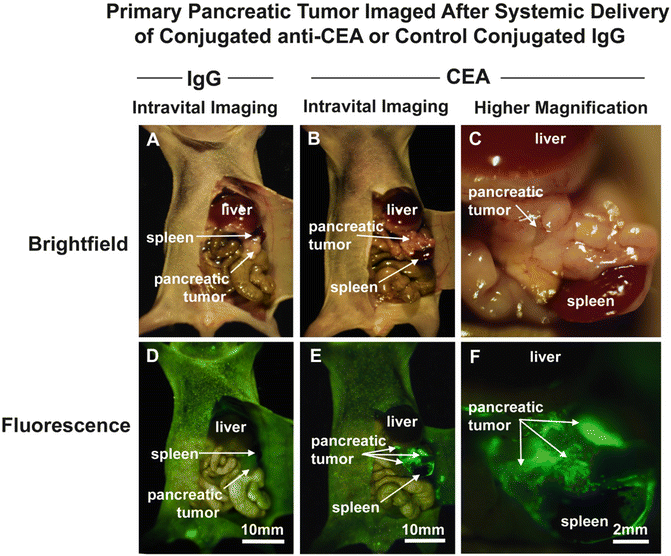
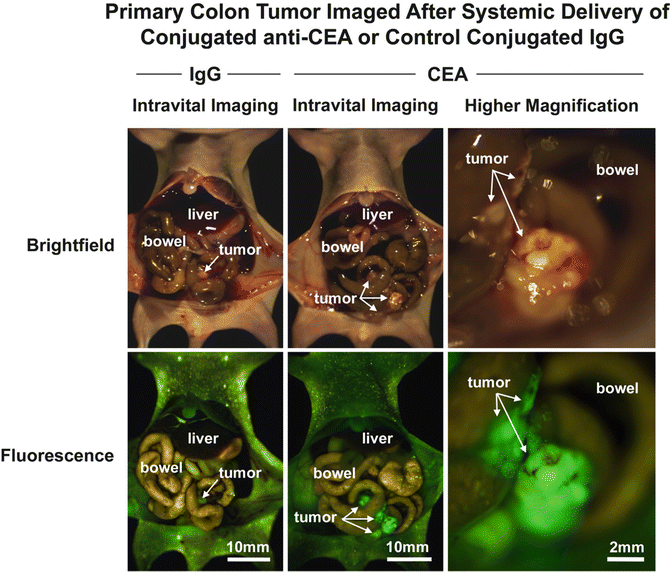

Fig. 22.1
Imaging of orthotopic human pancreas tumors in vivo had greatly improved primary tumor visualization at laparotomy. After treatment with fluorophore conjugated anti-CEA, mice with orthotopically-implanted BxPC-3 pancreatic tumors were imaged using both bright field (a–c) and fluorescence (d–f) illumination. Primary tumors were difficult to clearly distinguish under bright field imaging under both low (a, b) and high (c) magnification. In contrast, fluorescence illumination of anti-CEA-labeled tumors enabled identification of primary tumor (e, f), which was much more extensive than seen under bright field. Animals given conjugated non-specific IgG demonstrated no fluorescence signal in the orthotopic tumor (d). All tumors was confirmed by histology [12]

Fig. 22.2
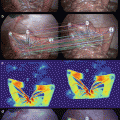
Fluorescence imaging of patient-derived orthotopic xenograft (PDOX®) human colon tumors under fluorescence illumination improved primary tumor visualization at laparotomy. Mice with orthotopically- implanted low-passage AC4104 colon tumors originally derived from a patient were imaged under both bright field (a–c) and fluorescence (d–f) illumination. Primary tumors labeled with conjugated anti-CEA had bright green fluorescence (e, f). Mice given conjugated control IgG had no fluorescence signal in the orthotopic tumor (d). All tumors were confirmed by histology [12]
Imaging Intra-abdominal Disseminated Tumor with Fluorescent Anti-CEA Antibody
Mouse models of intra-abdominal carcinomatosis of pancreatic and colorectal cancer were used to evaluate fluorophore-conjugated anti-CEA binding to these tumors in vivo. Animals were injected i.p. with human pancreatic (BxPC-3) or colorectal (Colo4104 or LS174T) cancer cells. After 1 week the mice were given a single 75 μg injection of Alexa Fluor 488-conjugated anti-CEA or IgG i.v. The mice were imaged 24 h later with the Olympus OV100 using both bright field and fluorescence illumination. At the time of imaging, these animals had very small peritoneal implants on the bowel and mesentery which were difficult to visualize using bright field imaging (Figs. 22.3a, b and 22.4a, b) but were very clearly visible under fluorescence illumination in mice given conjugated anti-CEA (Figs. 22.3c, d and 22.4c, d). The mice which received IgG had no visible fluorescence signal in their tumors (data not shown) [12].
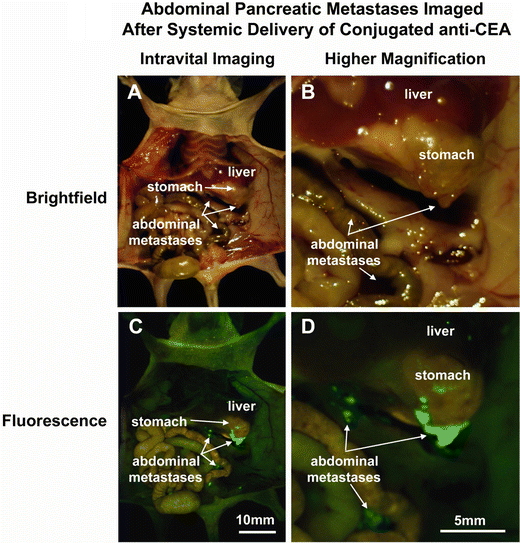
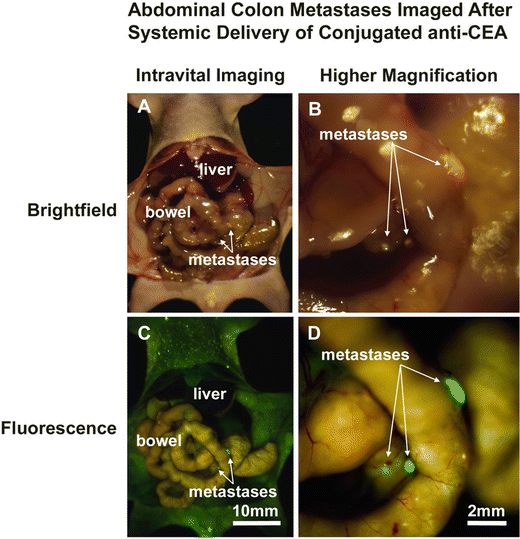

Fig. 22.3
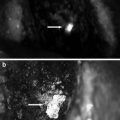
Fluorescence imaging of intra-abdominal metastases from human pancreatic cancer cell line BxPC-3 orthotopic models reveals greatly improved metastatic tumor visualization at laparotomy. Mice injected i.p. with BxPC-3 pancreatic cancer cells, were imaged using in bright field (a, b) and under fluorescence (c, d) illumination. Small metastatic implants on the bowel and mesentery were not seen with bright field imaging even under low (a) or high (b) magnification. In contrast, fluorescence illumination of anti-CEA-labeled tumors enabled easy identification of metastases (c, d) [12]

Fig. 22.4




Mice with metastatic human colon tumors had improved tumor visualization at laparotomy after injection of fluorophore-conjugated chimeric anti-CEA antibodies. Mice with intraperitoneally implanted Colo4104 colon cancer were imaged using both bright field (a, b) and fluorescence (c, d) illumination. The metastases were difficult to distinguish under bright field imaging under both low (a) and high (b) magnification. In contrast, fluorescence illumination of anti-CEA-labeled tumors enabled facile identification of metastases (c, d) [12]
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree