Introduction
Any discussion of chronic liver disease (CLD) would be remiss if a detailed description of hepatocellular carcinoma (HCC) were not included.
In 2018 primary liver cancer was projected to be the sixth most diagnosed and fourth most common cause of cancer death worldwide, with HCC making up 75%–85% of all primary liver cancers. CLD or cirrhosis is recognized as the most important precursor of HCC. Although only 1%–4% of people with cirrhosis develop HCC, 80%–90% of patients with HCC have underlying CLD or cirrhosis as a well-established risk factor.
Tumors in at-risk livers are predominantly HCC, but intrahepatic cholangiocarcinoma (ICC) has also been shown to have increased occurrence within the same at-risk population. , Therefore, as health care providers, we know where to look for those at risk for the development of these aggressive tumors—HCC and ICC—within the cirrhotic population.
HCC is a tumor of the liver hepatocytes. It develops by the process of hepatocarcinogenesis, which is the transformation of liver nodules from benign to malignant. Alternately, it can develop de novo or spontaneously in the liver. However, identification of spontaneous tumors is rare, and they have an unknown explanation. ,
The prognosis of patients with HCC is extremely poor, with a 5-year survival rate below 20%. Early detection of HCC is important as tumors detected in their early stages are more amenable to treatment, leading to increased survival rates and reduced disease-related mortality.
Liver imaging and the contrast-enhanced ultrasound (CEUS) algorithm, with a focus on its role in the determination of malignancy, is an important step in lesional analysis in all livers, including those with CLD and normal livers. However, recommendations for management and treatment of lesions in at-risk livers include the important classification system of the liver imaging reporting and data system (LI-RADS).
LI-RADS published on the American College of Radiology (ACR) website includes an algorithm created by a specialized team in the field of ultrasound (US) and CEUS to standardize performance, observation, and subsequent management of liver lesions. It includes US LI-RADS for detection of liver nodules and CEUS-LI-RADS for their characterization postdetection. The LI-RADS algorithm covers benign lesions, nonhepatocellular malignant tumors, and a spectrum of precursor and malignant hepatocellular tumors.
The focus of this chapter is to look at HCC and its development and the important role that US and CEUS can play as part of today’s aggressive management of HCC.
Bubble specific imaging
Following a standard gray-scale US, a lesion is located and optimally positioned for CEUS so it remains in the imaging plane during respiration. The contrast agents utilized for CEUS are lipid-encapsulated, gas-filled microspheres. The contrast agent and saline bolus are administered via intravenous injection. These bubbles have low toxicity and are thus introducible to the vascular system and remain for the duration of the diagnostic examination. The bubbles are sparse in circulation, therefore only a small amount of contrast (0.2–2.5 mL) is required per injection.
CEUS uses a contrast-specific software that has a dual display, with a low mechanical indexgray-scale image on one side and a microbubble-only image on the other. This gives superior sensitivity to microbubble signal detection. After adding a dual caliper, the lesion can be located on the gray-scale side and correlated with the precise location on the microbubble-only image (or vice versa).
Microbubbles are purely intravascular. They are approximately the same size as red blood cells and therefore can move through capillary beds, but their size does not permit them to pass through the vascular endothelium. They remain in the circulation without diffusing into the interstitium, unlike the contrast agents used in computed tomography (CT) and magnetic resonance (MR), which have a well-recognized interstitial phase.
As the microbubbles are purely intravascular, the contrast display reflects the volume of microbubbles exclusively within blood vessels in the organ and/or lesions. This allows for a sensitive depiction of tumor vascularity in all phases of contrast enhancement.
The unique capabilities of contrast-enhanced ultrasound important to liver imaging
For the diagnosis of HCC and its evolving nodules, CEUS has special imaging capabilities distinct from CT and MR that are invaluable. These include real-time scanning, high spatial resolution, narrow scan plane, and pulse-inversion imaging technique (i.e., the ability to create a microbubble-only image).
Microbubble imaging utilizes specialized techniques that alter the US pulses, including their number, phase, or amplitude. One of the earliest successful techniques is pulse-inversion imaging.
Pulse-inversion imaging
In pulse-inversion imaging, two pulses are sent from the US probe, the second pulse a mirror image of the first (180 degrees out of phase). For tissue that behaves in a linear manner, the sum of the pulses is 0, and imaging information from the tissue is subtracted. For echo with nonlinear components, such as oscillating microbubbles, the sum is additive. A signal is detected from the oscillating spheres and is subsequently enhanced. The linear liver echoes are subtracted, creating a microbubble-only image and an environment in which visualizing the smallest of vessels within an organ can be achieved. ,
Real-time scanning
Acquisition of images for CEUS utilizes dynamic real-time imaging performed at frame rates of about 10 per second, providing the highest temporal resolution available today in abdominal imaging. CEUS shows enhancement regardless of the arrival time of the microbubble contrast or the dynamic changes, including how fast the contrast arrives. Alternatively, MR and CT imaging have a predetermined timing protocol and therefore have static and fixed time points. Real-time scanning on CEUS overcomes this obstacle and shows vascular characteristics within the lesion that may be missed by either CT or MR, related to their timing or speed. Focal nodular hyperplasia (FNH) and hemangiomas with rapidly changing filling patterns are easily detected on CEUS. In particular, flash filling hemangiomas are of great importance when speaking about HCC as they are frequently found within cirrhotic livers.
Excellent spatial resolution
CEUS can observe contrast enhancement caused by the bubble oscillations as finite dots as small as 1–2 mm. This is a unique feature in comparison with all other contrast modalities. This allows CEUS to see blood flow within thin septations and small nodules, which is essential for the accurate classification of growing tumors.
Narrow scan plane
Thin slice thickness for CEUS improves characterization of septations and nodularity and detection of their enhancement.
Contrast-enhanced ultrasound arterial phase and portal venous phase
Approximately 20% of the liver’s blood supply comes from the hepatic artery and 80% from the portal vein. In liver contrast imaging, this creates the opportunity to observe an arterial phase (AP) involving the hepatic artery, and a portal venous phase (PVP) created by the portal vein. It is a unique environment for the observation of liver lesions, as tumors are mostly supplied by newly evolving arteries, and liver perfusion or parenchymal enhancement, the majority of which is supplied by the portal vein.
In CEUS, AP enhancement of a mass is assessed for its intensity relative to the enhancement of the background liver parenchyma. The intensity of intravascular contrast agents is determined by two important factors: (1) the volume of contrast in the blood vessels and (2) the rate of perfusion or time of uptake of contrast within the lesion or area of interest. Enhancement observations are generally classified into three groups in the AP relative to the background liver: isoenhancing, identical to; hypoenhancing, less than; and hyperenhancing, increased enhancement.
Arterial changes and documentation of the blood flow in evolving liver nodules are best recorded as cine clips and can be saved as high-quality movies in audio video interleave format. Movies are utilized to show the wash-in of the contrast agent from bubble arrival to peak enhancement. Still frames stored for future review are best acquired from the original movie clip. The added benefit of a microbubble-only image and pulse-inversion imaging gives CEUS the benefit of exceptional visualization of lesion enhancement without interference from background liver echoes, which have been subtracted from the microbubble-only image.
In addition to documentation of the arterial supply of identified nodules, subsequent imaging is focused on identification of wash-out in the PVP. Wash-out refers to a decline in the enhancement of the nodule to less than the enhancement of the adjacent liver parenchyma, following the initial AP enhancement. The process of wash-out is poorly understood, but it is believed that the normal venous supply of the liver is eliminated in tumor formation as a precursor and cause of wash-out.
Perfusion of the liver parenchyma and enhancement increase progressively through the AP and PVP lasting until 2 minutes. The start of the PVP is generally around the 40-second mark. In the late phase (LP) (>2 minutes) there is a steady decline in enhancement as the contrast leaves the liver parenchyma. The PVP and LP are best imaged by intermittent scanning at 1-, 2-, 3-, and 4-minute intervals to avoid bubble destruction by applying a consistent US beam.
An invaluable technique for PVP and LP, imaging is referred to as a liver sweep whereby the patient maintains full suspended inspiration while the probe is swept to include large portions of the liver in sagittal and axial planes. Wash-out areas show as small or large areas of decreased enhancement within the liver. The wash-out area can be optimally positioned using the contrast software, and another injection with contrast is done “on top” of the wash-out area to observe AP enhancement specific to the wash-out zone.
Contrast-enhanced ultrasound algorithm
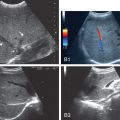
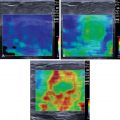
CEUS integrates the CEUS algorithm for diagnosis of liver lesions. It was described in the early work of Wilson and Burns published in 2006 and then expanded in 2017 by Burrowes et al. , It provides a comprehensive observational guide to the diagnosis and characterization of liver lesions, using their classic enhancement patterns ( Fig. 13.1 ).

The CEUS algorithm is important in lesion analysis in all livers including those with CLD and those with no risk factors. However, its ability to determine malignancy and direct lesion diagnosis relates directly to HCC and ICC (i.e., the focus of this chapter).
Although in reality we observe the enhancement patterns of lesions from the AP sequentially prior to the PVP, the most valuable way to interpret the liver lesions is to first determine if there is wash-out to assess for malignancy. Wash-out is the biggest and most important observation of malignancy. It can occur anytime, rapid in the AP but very often in PVP and in the LP. ,
Wash-out is invaluable for differentiating nonhepatocellular and hepatocellular malignancy and is classified according to its timing of onset and intensity : rapid vs. late wash-out and weak vs. marked.
Timing criteria for rapid wash-out are evaluated as a decrease in enhancement of the lesion before 1 minute, whereas in late wash-out the lesion begins to show decreased enhancement after 1 minute.
On all contrast-enhanced imaging, it is common to use the descriptive terms AP and PVP to define the time intervals following the contrast injection, with LP or delayed phase for images beyond 2 minutes. However, on CEUS, because of the dynamic real-time performance of the scan, there are multiple thresholds used in an algorithmic determination in which it is more suitable to use precise intervals in terms of seconds and minutes displayed on a clock.
Intensity description includes marked wash-out that must occur before 2 minutes following injection of the flush and appears as a black punched hole. Mild wash-out shows enhancement of the nodule less than the adjacent liver but continues to show evidence of bubbles within the nodule.
Late, weak wash-out after 1 minute is indicative of a hepatocellular malignancy or HCC. However, it is important to look for wash-out for up to 5 minutes, as delayed wash-out is an important feature to diagnose HCCs. Alternatively, rapid wash-out before 1 minute and/or marked wash-out are suggestive of a nonhepatocellular malignancy, including metastases, lymphoma, and cholangiocarcinoma.
Wash-out has been shown to occur in 97% of malignant lesions and 37% of benign lesions evaluated with CEUS. Of the benign lesions that showed wash-out, many can be accurately characterized in the AP.
Imaging with CEUS has value in the AP in which classic patterns contribute to diagnosis. The AP shows blood from the hepatic artery lasting to approximately 40 seconds. Timing begins at the start of the saline bolus, and the AP usually starts at 10–20 seconds and lasts up to 30–45 seconds following contrast injection. Its evaluation interval is from the arrival of the first bubble within the field of view until peak enhancement is achieved. The arrival of microbubbles in the AP is referred to as wash-in.
Rapidly changing dynamic patterns of AP enhancement and their observations are hugely important in diagnosis of benign lesions. Benign lesions show specific and reproducible AP enhancement patterns such as peripheral nodular enhancement. The direction of fill, such as centripetal and centrifugal filling, are also identifiable features of benign lesions. These characteristics are all easily recognized in CEUS during the AP.
The CEUS algorithm defines standard enhancement patterns for hepatocellular and nonhepatocellular malignancies. These enhancement patterns can vary considerably in the AP. Hepatocellular malignancies often have arterial phase hyperenhancement (APHE), which is globular or diffuse. More importantly, late- weak wash-out that occurs later than 1 minute is suggestive of HCC. Nonhepatocellular malignancies including cholangiocarcinoma, metastasis, and lymphoma also show variable AP enhancement patterns, including rim, diffuse, and hypoenhancement. However, rim APHE and strong wash-out before 1 minute are the most characteristic features for ICC.
The importance of liver imaging reporting and data system in at-risk livers
The CEUS algorithm is an important step in the classification of all lesions, from malignant to benign, in all livers. However, it is deficient for classification of lesions within a chronically diseased liver, because the spectrum of observations and the breadth of possibilities are much greater. LI-RADS provides detection of lesions within US LI-RADS and categorization of lesions according to a probabilistic scale within CEUS LI-RADS. All information on US and CEUS LI-RADS can be found on the ACR website.
Tumor detection hepatocellular carcinoma
Gray-scale surveillance and ultrasound liver imaging reporting and data system
Gray-scale US has become the cornerstone of surveillance programs for detection of tumors in patients with CLD. The recommended guidelines from American Association for the Study of Liver Disease (AASLD) suggest US every 6 months to fulfill this objective.
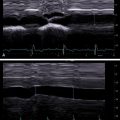
Surveillance US should include a thorough gray-scale US evaluation assessing for nodules and morphologic features of cirrhosis. Elastography is invaluable to measure liver stiffness, especially in patients with a liver nodule and unknown liver status and in those with moderate to severe fatty liver, allowing for a predetermination of the risk for potential HCC. Surveillance US requires excellent equipment and a high skill level for the performance of the scan. Familiarity with the wide range of background nodularity that can be shown in a cirrhotic liver is essential to allow for distinguishing the frequently larger and more conspicuous dominant nodules that require characterization with a contrast-enhanced imaging technique ( Fig. 13.2 ).

US LI-RADS helps make the distinction of a nodule from surveillance, necessitating further investigation using the three following criteria:
- 1.
US-1 negative, no nodule detected;
- 2.
US-2 subthreshold, an indeterminate US in which an observation of a nodule less than 10 mm is detected that is not definitely benign and may warrant short-term surveillance; and
- 3.
US-3 positive, a US study in which an observation of a nodule greater than or equal to 10 mm or a new thrombus in the vein is identified for which diagnostic contrast material–enhanced imaging or multiphase contrast imaging is recommended with either CT, MR, or US technique.
Tumor characterization hepatocellular carcinoma
Hepatocarcinogenesis
Prior to tumor characterization, knowledge about hepatocarcinogenesis and the associated change in vascular supply to evolving nodules is essential. Hepatocarcinogenesis is a multistep process of nodular evolution from a benign to a malignant nodule within a chronically diseased liver. HCC development is poorly understood but is believed to be caused by the inflammatory response created in CLD, inciting repeated cycles of cellular injury, death, and regeneration. This creates an insult to the normal liver cell, causing cirrhosis and possible genetic change to its DNA.
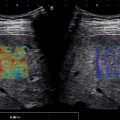
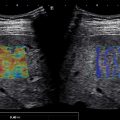
During hepatocarcinogenesis, liver hepatocytes create regenerative nodules (RNs) that enlarge and develop cellular atypia as they transform from low-grade to high-grade dysplastic nodules (DNs) before becoming HCC. Vascular changes accompanying hepatocarcinogenesis include the reduction of the normal paired arteries and hepatic veins, as well as neoangiogenesis, the formation of new blood vessels stimulated by an evolving tumor ( Fig. 13.3 ). Understanding the vascular alterations during hepatocarcinogenesis in evolving nodules and their gray-scale US appearance is essential to detect and characterize the many variations in morphology and vascularity that are possible in transforming nodules.