Primary tumor (T)a
TX
Primary tumor cannot be assessed
T0
No evidence of primary tumor
Tis
Carcinoma in situ
T1
Tumor 2 cm or less in greater dimension with less than two high-risk featuresb
T2
Tumor greater than 2 cm in greatest dimension or tumor of any size with two or more high-risk featuresa
T3
Tumor with invasion of maxilla, mandible, orbit, or temporal bone
T4
Tumor with invasion of skeleton (axial or appendicular) or perineural invasion of skull base
Regional lymph nodes (N)
NX
Regional lymph nodes cannot be assessed
N0
No regional lymph node metastasis
N1
Metastasis in a single ipsilateral lymph node, 3 cm or less in greatest dimension
N2
Metastasis in a single ipsilateral lymph node, more than 3 cm but not more than 6 cm in greatest dimension; or in multiple ipsilateral lymph nodes, none more than 6 cm in greatest dimension; or in bilateral or contralateral lymph nodes, none more than 6 cm in greatest dimension
N2a
Metastasis in a single ipsilateral lymph node, more than 3 cm but not more than 6 cm in greatest dimension
N2b
Metastasis in multiple ipsilateral lymph nodes, none more than 6 cm in greatest dimension
N2c
Metastasis in bilateral or contralateral lymph nodes, none more than 6 cm in greatest dimension
N3
Metastasis in a lymph node, more than 6 cm in greatest dimension
Distant metastasis (M)
M0
No distant metastasis
M1
Distant metastasis
Depth/invasion
>2 mm thickness
Clark level ≥ IV
Perineural invasion
Anatomic location
Primary site ear
Primary site non-hair bearing tip
Differentiation
Poorly differentiated or undifferentiated
Although the probability of regional and/or distant metastases is low, the likelihood increases with lesions that are extensive, poorly differentiated, and/or recurrent after prior treatment. Patients with SCC and perineural invasion (PNI) exhibit an increased risk of regional metastases [12]. Lymph nodes most often involved are in the parotid and/or neck. Andruchow and colleagues [13] have proposed a modification of the staging system for patients with positive nodes in these sites (Table 2).
Table 2
Clinical staging system for metastatic cutaneous squamous cell carcinoma to the parotid and/or neck [13]
Parotid | |
P0 | No clinical disease in the parotid |
P1 | Metastatic node up to 3 cm in diameter |
P2 | Metastatic node >3 cm and up to 6 cm in diameter or multiple nodes |
P3 | Metastatic node >6 cm in diameter or disease involving the facial nerve or skull base |
Neck | |
N0 | No clinical disease |
N1 | Single ipsilateral neck node up to 3 cm in diameter |
N2 | Single node >3 cm in diameter or multiple nodes or contralateral nodes |
Selection of Treatment Modality
The probability of cure for early-stage lesions is similar after surgery or RT. Therefore, the decision of which modality to employ depends on other factors, including function, cosmesis, patient age, cost, medical condition of the patient, treatment availability, and the wishes of the patient. Patients with advanced cancers are often best treated with surgery and adjuvant RT, if the functional and cosmetic outcomes are acceptable. In young patients, it is desirable to avoid RT because the late effects of irradiation progress gradually with time and, with very long-term follow-up, may be associated with a suboptimal cosmetic result.
Radiotherapy Alone
Resection of a relatively early-stage lesion of the eyelid, external ear, or nose may result in a significant cosmetic defect that would require a complex reconstruction. Patients with lesions in these locations are often better treated with RT, particularly if they are older and/or have a limited life expectancy. Advanced unresectable cancers, such as those with PNI with gross disease in the cavernous sinus, are treated with RT alone. Patients with advanced resectable cancers may be treated with RT alone depending on other factors, such as medical comorbidities.
Lesions on the scalp and anterior aspect of the lower leg over the tibia are located in areas where there is little tissue between the skin and underlying bone and are at increased risk for a bone exposure or necrosis after RT. Skin cancer in these locations is preferably treated surgically. Similarly the hands and feet generally do not tolerate high-dose RT well and skin cancers in these locations are better treated with an operation. Patients with connective tissue disorders, such as scleroderma, are at increased risk for a late complication after RT and, thus, this modality is best avoided.
Adjuvant Radiotherapy
Postoperative RT is added in situations where the likelihood of residual disease is relatively high, particularly if the probability of salvage of a local recurrence is relatively modest. Indications for postoperative RT include positive margins, PNI (particularly if it is symptomatic), multiple recurrences, and bone invasion. Some indications for postoperative RT are stronger than others, such as positive margins in patients with SCCs. Others, such as focal cartilage invasion with widely negative margins, are not strong indications in the absence of other adverse findings. Patients with BCCs on free skin that have been resected with a focally positive margin may be followed and treated only in the event of a subsequent recurrence.
Management of Regional Lymph Node Metastases
Patients who present with clinically negative regional nodes (cNo) and who receive definitive RT to the primary lesion receive elective nodal RT (ENI) if the risk of occult metastases is thought to be 15–20 % or higher. Patients with SCC and asymptomatic (incidental) PNI are in this category and would receive ENI. Patients with lower lip SCCs that involve the midline may have an unpredictable spread pattern to bilateral level I lymph nodes and would require bilateral ENI.
Skin cancer metastatic to the parotid nodes is managed in the same way that one would manage a high-grade parotid malignancy with superficial or total parotidectomy followed by postoperative RT [14, 15]. The facial nerve is preserved, unless it is necessary to resect it to achieve a gross total resection [16]. Although the risk of subclinical disease in the clinically negative nodes is probably 20 % or higher, the ipsilateral neck may be electively irradiated when the parotid is treated postoperatively. Preoperative RT is used for patients with borderline resectable metastases. RT alone is used for patients with unresectable disease and for those who are medically inoperable.
Cervical node metastases are managed in the same way that metastatic nodes are managed for primary mucosal carcinomas [17]. Neck dissection alone is sufficient for the patient with a solitary node with no extracapsular extension. Patients with more advanced disease receive postoperative RT. Depending on the location of the primary tumor and involved nodes, the probability of subclinical disease in the clinically negative parotid may be high and the parotid nodes should be considered for elective treatment.
Treatment Techniques
Primary tumor: The major RT techniques are as follows: (1) orthovoltage RT; (2) electron beam; (3) high-energy photons; (4) proton beam; and (5) interstitial brachytherapy. The majority of skin cancers are optimally treated with orthovoltage RT, such as 250 kVp X-rays. A customized lead shield is constructed to fit on the skin surface to collimate the beam. The advantages of orthovoltage RT compared with electrons are as follows: (1) the maximum dose is at the skin surface; (2) there is less beam constriction, both at the surface and at depth, so that smaller fields may be used; (3) the dose distribution is less likely to be adversely impacted by irregular surface contours, such as the nose and external ear; and (4) it is easier to shield the eye because there is less penetration through eyeshields, particularly at higher electron energies (see Fig. 1 and Table 3) [18]. Disadvantages of orthovoltage RT are that it has a higher exit dose compared with electrons, and there is a higher differential dose absorbed in bone and cartilage vs. soft tissue. Another disadvantage of orthovoltage RT is that most radiation oncology departments do not have orthovoltage equipment.
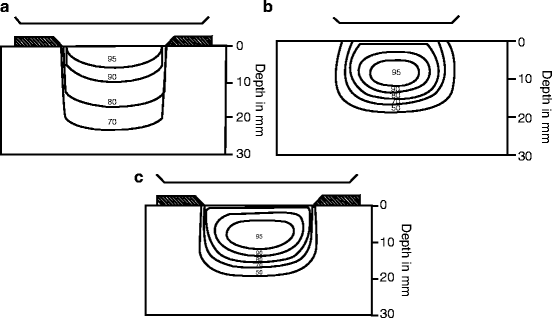

Fig. 1
(a) X-rays of 250-kVp (HVL 1.4 mm Cu) with secondary collimation of the phantom surface, source-to-surface distance (SSD) = 50 cm. Isodose %: 95, 90, 80, 70. (b) Electron beam of 6-MeV with secondary collimation 5 cm above the phantom surface (at the level of the electron cone). Source of collimator distance (SCD) = 95 cm. SSD = 100 cm. Isodose %: 95, 90, 80, 70, 50. (c) Electron beam of 6-MeV with tertiary collimation on the phantom surface. SSD = SCD = 100 cm. Isodose %: 95, 90, 80, 70, 50. Source: Mendenhall WM, Amdur RJ, Hinerman RW, Cognetta AB, Mendenhall NP. Radiotherapy for cutaneous squamous and basal cell carcinomas of the head and neck. Laryngoscope. 2009 Oct;119(10):1994–9
Structure (depth) | 250-kVp X-ray (HVL 1.4 mm Cu) (%) | Electron-beam energy (MeV) (%) | ||||||
|---|---|---|---|---|---|---|---|---|
6 | 8 | 10 | 12 | 14 | 17 | 20 | ||
Cornea (1 mm) | 10 | 18 | 37 | 64 | 75 | 93 | 98 | 102 |
Lens (8 mm) | 9 | 9 | 19 | 36 | 46 | 61 | 70 | 87 |
Retina (23 mm) | 10 | 19 | 22 | 22 | 21 | 23 | 25 | 29 |
Additional differences between photon beams and electron beams are that, for photons, as beam energy increases, surface dose decreases and exit dose increases. In contrast, for electrons, as beam energy increases, surface dose increases and exit dose increases. However, even for high energy electron beams, such as 20 MeV, one can only treat to a target depth of 4–5 cm before the dose falls off to a point where the tumor would be underdosed. Most skin cancers, if treated with electrons, are irradiated with 6–9 MeV beams, depending on the thickness of the lesion, with 0.5–1.0 cm of tissue equivalent material to assure an adequate surface dose. Our bias is to treat most skin cancers with orthovoltage RT except for scalp lesions where electron beam is employed to decrease the exit dose to the brain.
Regardless of whether electrons or orthovoltage irradiation are used, a lead mask is usually required to collimate the beam on the surface to obtain a sharp beam edge. Guidelines for selection of the dose-fractionation schedule are depicted in Table 4. These doses are increased by 10 % to account for the difference in radiobiological effectiveness (RBE) when used for megavoltage beams. The maximum suggested skin doses for palliation are shown in Table 5. Although fractionation schedules that include a small number of fractions are more likely to result in a suboptimal cosmetic outcome, these schedules are useful for treating elderly, infirm patients where more protracted schedules are not possible.
Orthovoltage dose (cGy) | Examples |
|---|---|
6,500 over 7 weeks | Large untreated lesion with bone/cartilage invasion or large recurrent tumor |
6,000 over 7 weeks | Large untreated lesion with minimal or suspected bone/cartilage invasion |
5,500 over 6 weeks |