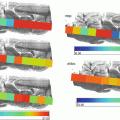
Fig. 1
Basic workflow of an imaging experiment with key focus areas; sample collection, sample preparation, choice of organic matrix , mode of application, and finally choice of mass spectrometer for analyses
Snap-freezing using liquid nitrogen at the point of collection is the most common method employed [1]. Cracking and fragmentation of the tissue may occur as the tissue cools down at different rates. To counteract this, Schwartz et al. (2003) recommend wrapping the tissue loosely in aluminum foil to stabilize the cooling [2]. On the other hand, Goodwin et al. (2008) mention that wrapping can distort the shape of the tissue, and therefore suggest a method of using ethanol or isopropanol at ≤−70 °C as a solution [3]. We obtained best results when floating a weighing boat containing our tissue onto liquid nitrogen. Fresh frozen tissue may remain frozen at −80 °C without signs of degradation for at least a year.
The alternative to fresh frozen preparations is to formalin-fix paraffin-embed tissue. It was thought that this form of preservation could limit the type of analysis carried out to peptide analysis alone, as the cross-linking in FFPE may prevent the detection of analytes such as metabolites, lipids, and pharmaceutical compounds, however this is not the case [4–6]. Drexler et al. (2007) reported the detection of small molecules in their study which indicates that FFPE may not limit compound analysis [7]. More recently, the Walch group have successfully imaged metabolites from FFPE tissue blocks; their method involves the deparaffinization of FFPE tissue and matrix coating by 9-aminoacridine prior to MALDI-MSI [8].
As the difficulties presented in analyzing FFPE tissues (due to complex cross-linking) are now successfully being overcome, further studies using the numerous samples in tissue banks would be of significant benefit [9]. Analysis would provide an insight into the histology of disease tissues with the potential to discover biomarkers by MALDI-MSI.
2 Materials
All solutions were prepared using ultrapure water (18 MΩ-cm at 25 °C) and analytical grade reagents with a purity of ≥99.5%. Reagents should be disposed of in compliance with health and safety regulations and in accordance with COSHH guidelines.
2.1 Materials for Mass Spectrometry Sample Preparation and Acquisition
- 1.
Dewar of liquid nitrogen .
- 2.
Carboxymethylcellulose (CMC) .
- 3.
Cork circles.
- 4.
Cryostat chuck.
- 5.
Surgipath X-tra adhesive precleaned micro slides .
- 6.
Ethyl alcohol, 200 proof, ACS reagent ≥99.5%.
- 7.
Chloroform ≥99.5%.
- 8.
Trypsin Gold, Mass Spectrometry Grade.
- 9.
Ammonium bicarbonate, BioUltra, ≥99.5%.
- 10.
Octyl-α/β-glucoside 10 mM solution (OcGlu).
- 11.
Methanol, CHROMASOLV®, for HPLC, ≥99.9%.
- 12.
Formalin solution, neutral buffered, 10%.
- 13.
Embedding cassettes.
- 14.
Xylene substitute.
- 15.
Embedding medium paraffin wax.
- 16.
Hydrogen peroxide solution (H2O2).
- 17.
Trizma base, ≥99.9%.
- 18.
α-Cyano-4-hydroxycinnamic acid (CHCA), ≥98% powder.
- 19.
Aniline, ACS reagent ≥99.5%.
- 20.
Acetonitrile, CHROMASOLV® Plus, for HPLC, ≥99.9%.
- 21.
Phosphorus red, 99%.
2.2 Reagents—Working Composition
- 1.
2% Carboxymethylcellulose .
- 2.
Ethanol solutions: Dilute 100% ethanol with ultrapure water to 90% and 70% (vol/vol).
- 3.
Ammonium bicarbonate 50 mM: Dissolve 0.2 g ammonium bicarbonate in 50 mL ultrapure water. Check and adjust pH to 8.0. Solution can be stored for 1 month.
- 4.
Trypsin solution 20 μg/mL: Add 1 mL ammonium bicarbonate (pH 8.0) directly to the trypsin 100 μg vial to give a 100 μg/mL stock solution. Agitate to ensure lyophilized trypsin is reconstituted. For the 20 μg/mL working solution, pipette 200 μL of the stock solution into a sterile 1.5 mL snap top Eppendorf and add 800 μL ammonium bicarbonate (pH 8.0).
- 5.
Trypsin solution 20 μg/mL containing detergent 0.5%: Add 5 μL detergent solution (10 mM) to 995 μL trypsin solution 20 μg/mL.
- 6.
Ethanol solutions for paraffin embedding: Dilute 100% ethanol with ultrapure water to 70%, 80%, and 95% (vol/vol).
- 7.
MeOH: H2O2 3% solution: Mix together 48.5 mL and 1.5 mL H2O2.
- 8.
Tris–HCl buffer (1 L): Completely dissolve 1.21 g Trizma base in 1000 mL of ultrapure water. Adjust pH to 9.0 with 1 M NaOH or HCl as required (solution can be stored in a glass duran for up to 6 months).
- 9.
MALDI matrix solution: Prepare 5 mg/mL CHCA in 50% (vol/vol) acetonitrile and 0.5% (vol/vol) trifluroacetic acid in ultrapure water . Add equimolar amounts of aniline to the CHCA (i.e., 1 mL of 5 mg/mL CHCA solution contained 2.4 μL aniline). Sonicate in a sonic water bath for 5–10 minutes until matrix crystals have fully dissolved. Matrix containing aniline should be prepared fresh on the day of the experiment and not stored.
- 10.
Phosphorus red calibrant: 10 mg/mL in 100% acetone. Vortex for 10–15 seconds. N.B. phosphorus red may not dissolve entirely.
3 Methods
3.1 Cryo-conservation of Tissue
- 1.
Arrange the excised organ/biopsy into the correct orientation onto a regular weighing boat.
- 2.
While wearing appropriate PPE, float the weighing boat containing the tissue on liquid nitrogen (see Note 1 ).
- 3.
Take ~50 μL of 2% CMC and attach the frozen tissue to the cork circle, ensuring the tissue is level and in the correct orientation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree