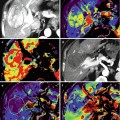
Fig. 47.1
Muscle-invasive bladder cancer. (a) Coronal T2-WI shows extravesical mass on the left bladder wall. (b, c) Gadolinium-enhanced image shows cT3 tumor with extension into the extravesical fat
47.2.2 DW-MRI in Bladder Cancer
DW-MRI is a noninvasive, functional imaging technique that was developed by quantifying the microscopic mobility of water molecules in the tissues. Using this unique technique, malignant lesions usually show high signal intensity because of their higher cellularity, tissue disorganization, and decreased extracellular space, all of which restrict water diffusion [6, 7]. The extent of water molecule diffusion is quantified by calculating the apparent diffusion coefficient (ADC) value, and DW-MRI has recently begun to be used to detect, stage, and characterize urinary system tumors [8].
The feasibility of using DW-MRI to detect bladder cancer was first reported by Matsukiet al. Using high b-value DW-MRI, bladder tumors appear as hyperintense lesions compared to the hypointense signals of the surrounding tissues, including the urine and normal tissues [9] (Figs. 47.2 and 47.3). A high degree of sensitivity and specificity in the detection of bladder cancer has been shown. Abou-El-Ghar et al. reported the sensitivity, specificity, and accuracy of DW-MRI for detecting bladder cancer in patients with gross hematuria were 98, 92, and 97 %, respectively [10]. Ceylan et al. reported that DW-MRI had 90 % sensitivity, 93 % specificity, and 91 % accuracy to detect bladder cancer in 59 patients who had gross hematuria or who were being followed up after previous bladder cancer [11]. Studies comparing ADC values have consistently shown restricted diffusion in bladder tumors; the ADC values of bladder tumors were significantly lower than those of the surrounding structures [9, 12]. Kobayashi et al. recently showed, in a prospective study that included 121 patients with bladder cancer, that better interobserver agreement was attained when bladder cancer was assessed using DW-MRI compared to T2-WI but that the ability to detect cancer was equivalent between these two imaging sequences [13].
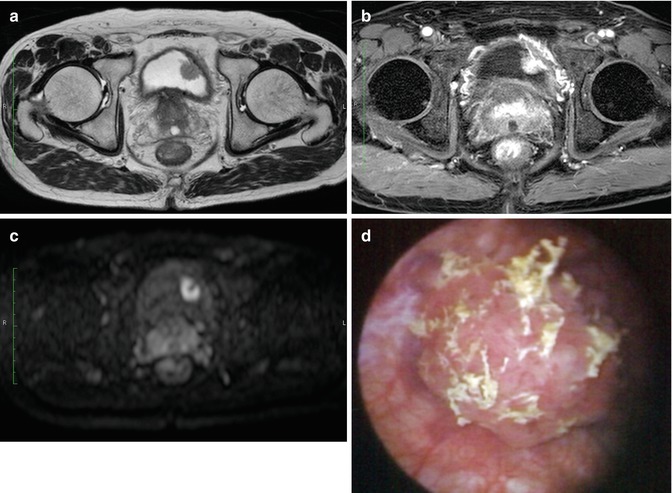
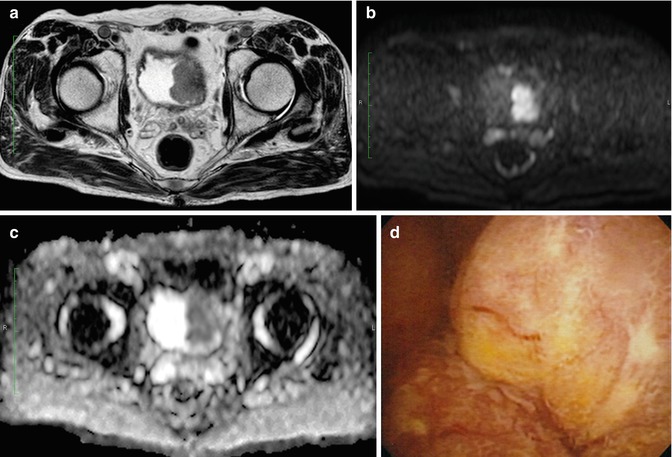

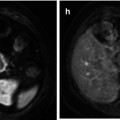
Fig. 47.2
Non-muscle-invasive bladder cancer. (a) T2-WI shows a papillary tumor on the left wall of the bladder. (b) Gadolinium-enhanced image shows vessels feeding the tumor in the stalk. (c) DW-MRI displays the tumor as a high-signal mass, while the stalk of the tumor is not shown. (d) Cystoscopic view of this papillary pedunculated tumor

Fig. 47.3
Muscle-invasive bladder cancer. (a) T2-WI shows a solid tumor focally disrupting the muscle layer. (b) DW-MRI displays the tumor as a high-signal mass without submucosal components. (c) The apparent diffusion coefficient map shows restricted diffusion in the tumor. (d) Cystoscopic view of this sessile tumor
The feasibility of using DW-MRI in staging bladder cancer has been shown based on the signal differences of the different tissues: hypointense signals in the submucosal layer, hyperintense signals in the tumor, and intermediate signal intensity in the intact bladder wall [14, 15]. In one study that included 106 patients with bladder cancer, DW-MRI was able to differentiate non-muscle-invasive tumors from invasive tumors with 63.6 % accuracy [14]. Another study that included 40 patients with 52 bladder cancers reported that the combined use of DW-MRI with T2-WI increased the bladder cancer staging accuracy [15]. The staging accuracy increased in this study from 67 % using T2-WI alone to 88 % using both methods. These two studies are unique because staging evaluation was made based on only signal shape obtained using DW-MRI.
The underlying mechanism whereby the ADC value reflects the tumor characteristics is generally thought to be associated with tumor cell morphological features, including dense cellularity and large cellular size; however, a recent study suggests that there is a correlation between the bladder cancer ADC value and cell proliferative potential, which is evaluated using the Ki-67 labeling index [6, 16]. Therefore, quantitative evaluation of DW-MRI appears to reflect biological properties of tumors. In urothelial cancer, ADC values can be associated with the histological grade. Takeuchi et al. reported a prospective study showing that the ADC value of grade 3 tumors was significantly lower than that of grade 1 and 2 tumors [15]. Several papers also reported similar results showing an inverse correlation between the ADC value and the histological grade [13, 17]. However, ADC values showed a substantial overlap in high- and low-grade tumors.
As a biological marker, the ADC value can predict not only histological grade but also clinical aggressiveness and metastatic potential of the bladder cancer. Kobayashi et al. reported that the ADC values were inversely correlated with both histological grade and T stage, and they also showed an accuracy of 87 % using DW-MRI to distinguish clinically aggressive disease, including muscle-invasive disease or high-grade T1 bladder cancer, from others [13]. Rosenkrantz et al. reported the potential of the ADC value in assessing the metastatic potential of localized high-grade bladder cancer [13]. In a small study that included 17 patients with histologically confirmed high-grade bladder cancer, the ADC value was lower in the patients with invasive high-grade tumors and metastatic disease than it was among those without metastases [18]. The ADC value can serve as an objective and quantitative biomarker to predict the clinical aggressiveness of bladder cancer.
Because DW-MRI examination provides comprehensive information on characteristics of bladder cancer, this functional imaging is expected to be a biomarker for predicting and monitoring treatment response [16, 19]. Chemoradiotherapy (CRT)-based bladder preserving strategy for localized muscle-invasive bladder cancer can be applied only if a complete response is obtained [20]. In a small cohort of patients with localized muscle-invasive bladder cancer who were uniformly treated with induction CRT, the feasibility of DW-MRI was shown in predicting the sensitivity and monitoring the effect of the induction treatment [16, 19]. A lower ADC value before CRT was shown to be a significant predictor of CRT sensitivity, presumably because proliferating bladder cancer has a high level of sensitivity to CRT [16]. In addition, complete absence of an abnormal signal using post-CRT DW-MRI indicated that a complete response was achieved, with a specificity of 92 % and an accuracy of 80 %; these parameters were significantly better than those of T2-WI and DCE-MRI, even though the sensitivity for the microscopic residual cancer was relatively low (57 %) equivalent to the sensitivity of T2-WI and DCE-MRI [19].
47.2.3 DW-MRI in Upper Urinary Tract Cancer
The role of imaging for identification and characterization of upper urinary tract cancer (UUTC) is clinically more important compared to that of bladder cancer because of increased invasiveness and inaccuracy of biopsy under ureteroscopy [21, 22]. A combination of morphological imaging with urinary cytologic examination has been widely used for diagnosis of UUTC. Recently, the high sensitivity and high accuracy of multidetector CT urography for detecting UUTC have been reported with a sensitivity of 89 % [23, 24]. However, overuse of iodinated radiocontrast media should be avoided for the patients who need to undergo nephroureterectomy or chemotherapy.
Technological advances make DW-MRI available for abdominal lesions, and DW-MRI in free breathing conditions depicts the UUTC. Since the first report from Yoshida et al. demonstrating the feasibility of DW-MRI for detecting UUTC, several studies have confirmed the excellent diagnostic performance of DW-MRI [25–30] (Figs. 47.4 and 47.5). In a prospective study that included 76 patients with suspected UUTC, Yoshida et al. reported that DW-MRI differentiated between 49 patients with UUTC and 27 patients with benign conditions, with 92–93 % sensitivity, 81–96 % specificity, and 89–93 % accuracy [30]. Use of DW-MRI with T1- and T2-WI improved the sensitivity and accuracy, while the specificity did not change. Akita et al. also reported that sensitivity improved from 88 to 98 % when DW-MRI was added to T2-WI in the detection of UUTC [25]. Unlike bladder cancer, which has consistently shown an association between the ADC value and histological grade, this correlation remains controversial for UUTC; two larger studies reported the correlation, while the others did not [25–27].
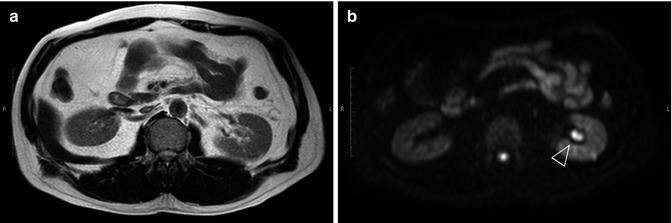
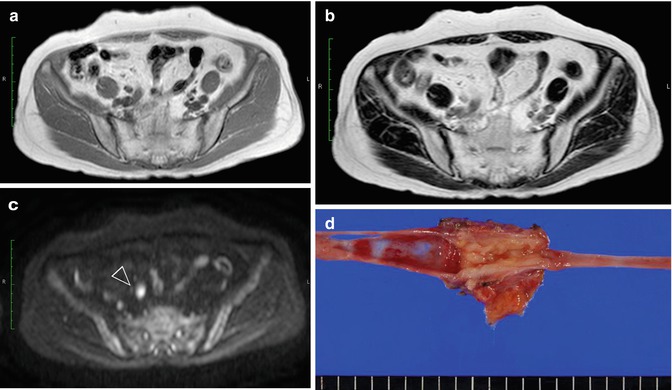

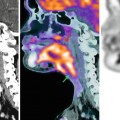
Fig. 47.4
Left renal pelvic cancer. (a) Using T2-WI, the tumor is displayed as a signal that is almost iso-intense with renal parenchyma. (b) Using DW-MRI with a b-value of 800 s/mm2, renal pelvic cancer (arrowhead) is displayed as a bright white signal compared to the surrounding tissues

Fig. 47.5
Right ureteral cancer. Using T1-WI (a) and T2-WI (b), the signal of ureteral tumor is iso-intense with that of the surrounding vessels. Using DW-MRI, the high-intensity signal of ureteral cancer (arrowhead) is easily identified (c). (d) Gross photograph of nephroureterectomy specimen
ADC value could be a potential biomarker reflecting tumor proliferative potential. ADC value was inversely correlated with Ki-67 labeling index in UUTC as well as bladder cancer [31, 32]. Thus, preliminary report also showed the usefulness of ADC value for preoperative prognostic risk stratification. A lower ADC value was associated with a shorter cancer-specific survival in UUTC patients treated with nephroureterectomy alone [32].
47.2.4 PET/CT in Bladder Cancer
18F-FDG-PET/CT is widely used in the evaluation of various abdominal lesions. The application in staging primary bladder cancer has been still limited due to the activity of 18F-FDG excreted through the urinary system. To overcome this problem, diuretic administration and oral hydration are used. A sensitivity and specificity of 18F-FDG-PET/CT using diuretic and oral hydration to detect primary tumor in patients with invasive bladder cancer were reported to be 54–87 % and 25–100 % [33–35]. Good staging accuracy of 18F-FDG-PET/CT has been reported in detecting lymph node and distant metastases [36–39] (Fig. 47.6). Apolo et al. investigated 47 patients with bladder cancer and found that the sensitivity and specificity for metastatic disease were 81 and 94 %, respectively [36]. This study evaluated the clinical physicians’ assessment based on the national oncologic PET registry questionnaire and reported the direct benefit from 18F-FDG-PET/CT over that achievable using routine MRI or CT. Kibel et al. evaluated the value of preoperative 18F-FDG-PET/CT for detecting lymph node metastasis in 42 patients with cT2N0M0 bladder cancer based on CT and bone scintigraphy findings [37]. While 18F-PET/CT showed false-positive findings (specificity, 94 %) in 2 of the 32 patients with negative lymph node metastasis, 18F-PET/CT detected metastases in 7 of 10 patients whose lymph nodes were shown to have metastasis using pathologic examination (sensitivity, 70 %). The investigators also reported that the survival rate after 18F-FDG-PET/CT scan was significantly lower in the patients who had a positive 18F-FDG-PET/CT compared to those with a negative 18F-FDG-PET/CT.
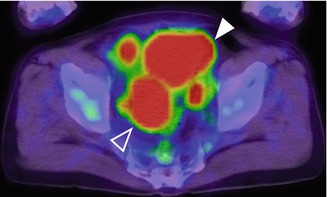

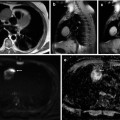
Fig. 47.6
Axial 18F-FDG-PET/CT image of bladder cancer with right external and left obturator lymph node metastases. A high level of tracer uptake is seen in the metastatic nodes. The accumulated intense signal of excreted urine (arrowhead) and bowel content (white frame arrowhead) are also shown
To improve the accuracy in detecting bladder cancer, non-FDG radiotracers that have minimal urinary excretion, including 11C-choline, 11C-acetate, and 11C-methionine, have been evaluated [40–43]. These tracers have the potential to improve the diagnostic accuracy of bladder cancer. However, these tracers are not readily available, and further study is required to evaluate them.
47.3 Functional Imaging of Adrenal Gland Tumors
47.3.1 DCE-MRI in Adrenal Gland Tumors
DCE-MRI proved its value to differentiate benign and malignant adrenal tumors due to different enhancement patterns [44]. The presence of a homogeneous capillary blush and rapid washout are characteristic of adrenal adenomas [45]. In clinical practice, the role of DCE-MRI in the distinction of adrenal masses is complementary to chemical-shift MRI, being especially useful in cases of atypical adenomas [46].
47.3.2 DW-MRI in Adrenal Gland Tumors
Using DW-MRI, normal adrenal glands and adrenal tumors show high signal intensity and are easily recognized when compared to morphological images (Figs. 47.7 and 47.8). However, several studies did not show the usefulness of DW-MRI in characterizing adrenal gland tumors [47–49]. Considerable overlap of ADC values was detected between nonfunctioning adenoma, aldosteronoma, and Cushing’s adenoma [49




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree