Fig. 1
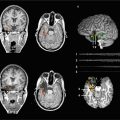
The state-of-the-art preoperative multimodality MR imaging in a right glioblastoma affecting the basal ganglia, insula, and inferior frontal lobe as visualized on standard MR sequences (a, upper row). Advanced MR imaging (b, lower row) revealed disintegration of white matter architecture/alterations in fractional anisotropy on color-coded DTI maps (left), increased relative blood volume on dynamic susceptibility contrast-enhanced (DSCE) perfusion imaging (middle), and right language dominance on BOLD fMRI (right) in this left-handed male patient (Color figure online)
2 Blood-Oxygenation-Level-Dependent Functional MRI (BOLD fMRI)
Enhanced synaptic activity resulting from stimulation of neurons leads to a local increase in energy and oxygen consumption in functional areas. The following local hemodynamic changes transmitted via neurovascular coupling are measured by fMRI with high spatial accuracy:
Increase of regional cerebral blood volume (rCBV)
Increase of regional cerebral blood flow (rCBF)
Relative increase of oxyhemoglobin in capillaries and venous blood
In principle, fMRI measurements can be accomplished with different techniques; BOLD fMRI being the most frequently used in human brain is therefore often referred to as the standard technique (Thulborn et al. 1996; Thulborn 1998, 2006). Bolus tracking measures rCBV making use of external contrast agents (Belliveau et al. 1991), and spin tagging assesses rCBF using arterial blood for intrinsic contrast (Detre et al. 1992). In contrast, BOLD technique (Ogawa et al. 1990, 1992, 1993) takes advantage of the different magnetic properties of oxygenated (oxy-Hb) and deoxygenated (deoxy-Hb) hemoglobin to generate an image contrast. Paramagnetic deoxy-Hb produces local field inhomogeneities in a measurable range for MRI resulting in a signal decrease in susceptibility-weighted MR sequences (T2*), whereas diamagnetic oxy-Hb does not interfere with the external magnetic field. Specific neuronal stimulation augments local cerebral oxygen consumption, which initially results in a decrease of oxy-Hb and an increase in deoxy-Hb in the functional area. To provide the active neurons with oxygenated blood, perfusion (rCBF, rCBV) in capillaries and draining veins is increased within several seconds. This mechanism not only equalizes the initial decrease of local oxy-Hb concentration but even overcompensates it (Fox and Raichle 1986). Deoxy-Hb is progressively washed out which is reflected by a reduction of local field inhomogeneity and a raise of BOLD signal in T2*-weighted MR images (Turner et al. 1991).
BOLD measurements are currently exerted with ultrafast single-shot echo-planar-imaging (EPI) sequences as gradient echo (GRE) or spin echo (SE) (Stippich et al. 2002a, b). GRE sequences usually obtain higher BOLD signal predominantly from venous origin, whereas SE sequences also measure lower BOLD signals arising from the capillary bed in brain parenchyma (Hulvershorn et al. 2005a, b). Temporal resolution in paradigms with a blocked or parametric design corresponds to the chosen block duration but could be substantially improved (<100 ms) by the introduction of event-related measurements (Buckner et al. 1996). FMRI, however, cannot attain the temporal resolution of electroencephalography (EEG) (Berger 1929; Gevins 1995; Gevins et al. 1995) or magnetoencephalography (MEG) (Hari and Ilmoniemi 1986; Hämäläinen et al. 1993) but lacks the need for a complicated model-based calculation for source localization and offers higher spatial precision due to a direct correlation with the surface anatomy. Signal intensity and realizable spatial resolution vary with the magnetic field strength. MR scanners with magnetic field strengths below 1.0 Tesla (T) are not suitable for clinical functional BOLD imaging. The broadly available 1.5 T scanners allow reliable measurements of cortical activation provided that powerful gradient systems (possibly >30 mT/m) are on hand, while high-magnetic-field scanners with 3 T or above even permit functional imaging of subcortical structures and the brain stem (Thulborn 1999; Zambreanu et al. 2005). Despite excellent SNR and spatial resolution, the application of fMRI at field strengths 7.0 T or above has to be considered experimental (Goa et al. 2014; Hua et al. 2013; Huber et al. 2014). This is mainly due to technical and logistical difficulties as well as to the very limited availability of ultrahigh-field MR imagers in a clinical setting. Advantages of fMRI over positron emission tomography (PET) (Mazziotta et al. 1982; Raichle 1983; Fox et al. 1986) or single photon emission computer tomography (SPECT) (Holman and Devous 1992) – other methods measuring brain activity indirectly with lower spatial/temporal resolution (glucose or oxygen metabolism, blood flow/perfusion changes) – are its noninvasiveness, lack of radiation, reproducibility, and broad availability of clinical scanners.
Task-based presurgical BOLD fMRI represents the best established and validated clinical application of fMRI, whereas resting-state fMRI should still be considered under initial clinical investigation. Due to the intrinsically low SNR, it is mandatory to perform multiple repeated stimulations during each task-based fMRI measurement in order to obtain robust BOLD signals. Statistical correlation of BOLD signal time courses with the chosen stimulation protocol (paradigm) enables the identification of those brain areas that show hemodynamic changes in synchrony with the task. A prerequisite for this is a further processing of fMRI data, which is normally done after the measurements with freely available or commercial software (Cox 1996; Friston 1996; Gold et al. 1998; Roberts 2003). However, not all of these programs have been certified for medical use. Nowadays, most manufacturers of clinical high-magnetic-field scanners offer options for an online processing of fMRI data (real-time fMRI) (Fernandez et al. 2001; Moller et al. 2005; Posse et al. 2013). Since the functionality and the statistical calculations used vary considerably between the different softwares, the appropriate choice largely depends on individual criteria and preferences. Basically, any data processing software for fMRI should at least offer image alignment, motion correction, temporal and spatial data smoothing, multiple statistical tests to assess functional activation, and options for spatial normalization (Stippich et al. 2002a, b). Furthermore, each fMRI software used for neurosurgery or radiation treatment should provide precise and reliable tools for superimposing functional on morphological images, for the integration of fMRI with DTI data, and for data export (e.g., into neuronavigation systems).
This textbook has its traditional focus on presurgical functional neuroimaging. Today, presurgical task-based fMRI is the most widely used modality and is commonly combined with DTI and DTT to visualize both functionally important cortical areas and white matter tracts. Hence, we have now added essential information on this complementary MR modality but keep the focus strictly on pre- and intraoperative applications in patients with brain tumors and epilepsy. A detailed description of diffusion MRI is beyond the scope of this book – for this purpose, we refer the reader to the extensive literature available.
3 Diffusion Tensor Imaging (DTI) and DTI Tractography (DTT)
Diffusion MR signal mainly originates from protons that move in the extracellular space of biological tissues (Brownian motion) (LeBihan 2006, 2013; LeBihan and Johansen-Berg 2012). Within a given voxel, the diffusion may be fully free and undirected (isotropic) or directed (anisotropic) due to barriers preventing proton movement (e.g., myelinated axons). DTI is capable to detect such anisotropic diffusion (Basser et al. 1994; LeBihan et al. 2001), but for this end diffusion must at least be measured in six different directions. Anisotropic diffusion can then be modeled mathematically using a 3D Gaussian probability function from which a 3 × 3 matrix is calculated, named diffusion tensor. The diffusion tensor is characterized by eigenvalues and eigenvectors and indicates the main orientation of diffusion within a voxel. The fractional anisotropy (FA) quantifies such directed diffusion. Isotropic (undirected) diffusion corresponds to an FA value of 0 which translates graphically to a sphere. In contrast, an FA value of 1 reflects the opposite extreme, i.e., a totally directed (anisotropic) diffusion which would graphically translate to a line but does not occur in biological tissues – thus, the typical shape of the FA is an ellipsoid.
Myelinated axons are lipophilic and represent diffusion barriers for hydrophilic protons that travel perpendicularly. In turn, this results in a preferred orientation and higher velocity of diffusion along such barriers. Thus, DTI reflects the white matter architecture and the course of functionally important fiber bundles rather indirectly by measuring the anisotropic diffusion of protons between and along axons. Basically, DTI fiber tracts are reconstructed by following and connecting the predominant directed diffusion across adjacent voxels. Standard DTI measurements and most mathematical algorithms employed for analyzing DTI data have limitations when different orientations of diffusion need to be differentiated within a voxel, when crossing fibers need to be reconstructed, or when diffusion anisotropy is affected by the brain’s pathology, e.g., by tumor invasion or edema (Cortez-Conradis et al. 2013; Kallenberg et al. 2013; Kleiser et al. 2010; Stadlbauer et al. 2010). At the expense of scanning/processing time, more advanced MR techniques enable the assessment of the contribution of the differential intravoxel anisotropy (diffusion spectrum imaging, q-ball imaging, HARDI, etc.) or the employment of a more sophisticated (e.g., probabilistic or advanced deterministic) modeling for tractography (Bauer et al. 2013; Kuhnt et al. 2013a, b; O’Donell et al. 2012). In general, the quality of DTI images depends on the number of directions measured, the number of averages used, and the spatial resolution chosen, which are all inversely related to scanning time.
4 Presurgical fMRI and DTI
Presurgical fMRI and DTI measurements are carried out to facilitate function-preserving and safe treatment in patients with brain tumors and epilepsy by localizing and lateralizing specific brain functions, functionally important axonal connections, or epileptic activity, noninvasively (Bick et al. 2012; Dimou et al. 2013; Centeno and Carmichael 2014). This diagnostic information cannot be obtained from morphological brain imaging alone or from invasive measures prior to treatment.
Consequently, presurgical fMRI and DTI are always performed in individual patients to achieve a functional diagnosis. This differs fundamentally from applications in basic neuroscience research where normal or altered brain function is usually investigated in group studies to better understand physiological or pathological conditions in general. A direct contribution to the patient management is not required. In contrast, for clinical diagnostic applications, the experimental setup as well as the processing and evaluation of the data need to be adapted to the clinical environment so that patients – who may present with neurological or cognitive deficits – can be examined successfully in a standardized way. Still, in clinical fMRI and DTI, this is typically achieved via local experts, individual routines, and own validation (Pillai 2010). The novel applications of resting-state fMRI (RS-MRI) may have practical advantages, as an active cooperation of the patient is not required (Lang et al. 2014). Uncooperative, sedated, or anesthesized patients or children who are not able to perform task-based fMRI properly may profit from RS-MRI. Potentially, RS-fMRI could also be applied intraoperatively (Nimsky 2011). The available data on presurgical RS-fMRI are very limited, however, and therefore do not justify to draw any firm conclusion on its clinical feasibility and validity, yet.
During the last years, more and more devices and software solutions have become commercially available that facilitate the clinical application of fMRI. These products are mainly dedicated to stimulation and data processing. Moreover, first attempts have been made to define robust clinical applications for task-based fMRI. To this end, paradigms have been proposed and published online by the American Society of Functional Neuroradiology (for details, please visit www.asfnr.org/paradigms.html). At least in the USA, CPT codes have been established for reimbursement of clinical fMRI examinations. It is important to note, however, that fMRI and DTI cannot be considered as fully standardized methods routinely used in clinical diagnostic neuroimaging. Hence, depending on the local situations, it may still be required to perform presurgical fMRI and DTI examinations in the framework of scientific studies until all hard- and software components used for medical application have been certified and official recommendations or guidelines have been issued by the relevant national medical association. Until then, individual routines and standards still need to be established for data acquisition, processing, and evaluation, as well as for the medical interpretation and documentation of clinical fMRI and DTI findings. For a meaningful clinical application, a profound knowledge of the specifications of the different software packages used, of possible sources of errors and imaging artifacts, and of relevant limitations of the techniques employed is indispensible. As a basic principle, presurgical fMRI and DTI examinations should be performed, evaluated, and interpreted by trained and experienced physicians with particular expertise in this area, since careless use of this very promising technique could endanger patients.
There are numerous studies available suggesting a high reliability of fMRI to localize the different cortical representations of the human body within the primary motor and somatosensory cortices (Fig. 2) as well as the motor and sensory language areas (Fig. 3) prior to brain surgery, if indications and limits of the method are considered. The same holds true for the noninvasive determination of the dominant brain hemisphere for language function.
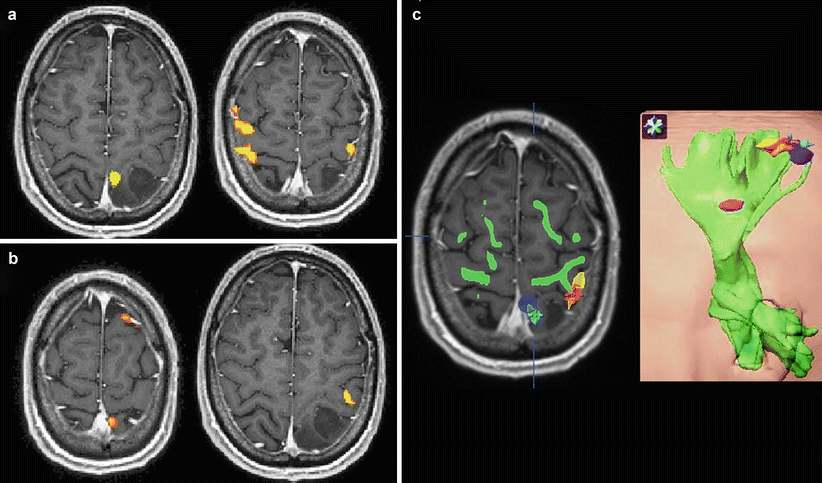
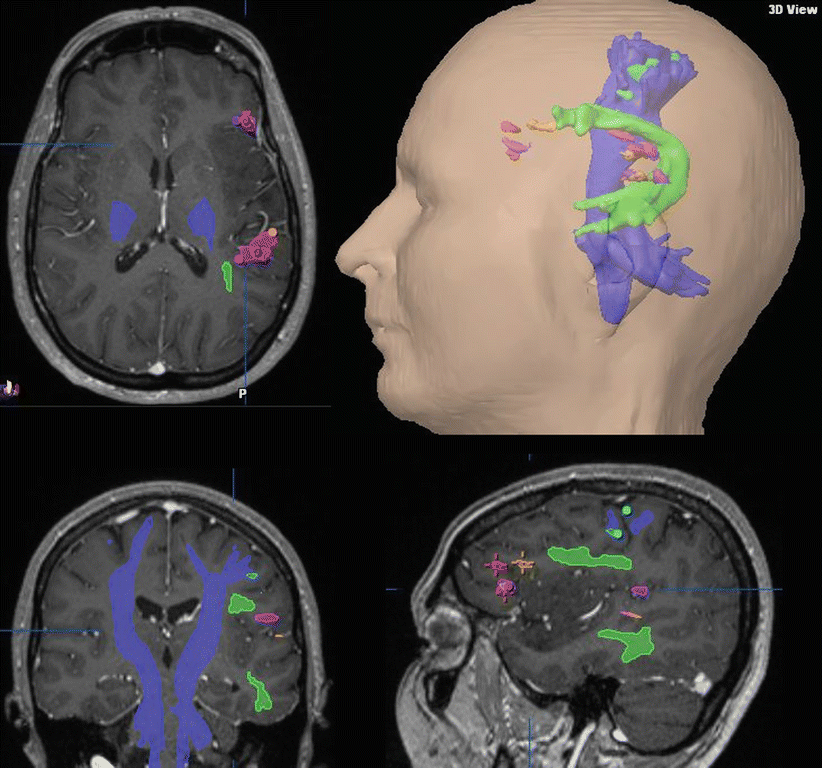

Fig. 2
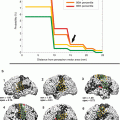
Preoperative fMRI somatotopic mapping of the motor (a) and somatosensory (b) homunculus and DTI tractography of the corticospinal (pyramidal) tract in a symptomatic patient with a left parietal astrocytoma. Voluntary movements of the toes and fingers on the right side were used for motor mapping, and fully automated pneumatically driven tactile stimulation of digits 1 and 2 to the right foot and hand, respectively, was employed for somatosensory stimulation. Of note is the enhanced co-activation of the premotor and primary hand representations in the right hemisphere during voluntary right hand movements as an fMRI indicator for tumor-associated neuroplastic reorganization. Integration of the abovementioned different functional body representations (foot motor (dark blue), foot somatosensory (light blue), hand motor (yellow), hand somatosensory (orange), tongue motor (purple)) and of the pyramidal tract (green) into a 3D data set for functional neuronavigation (c, left), 3D surface view (c, right) (Color figure online)

Fig. 3
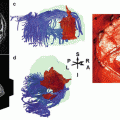
Preoperative fMRI language mapping of the motor (Broca) and sensory (Wernicke) language areas and DTI tractographies of the arcuate fascicle (green) and pyramidal tract (purple) integrated into the T1-weighted post-contrast 3D MRI used for functional neuronavigation in a female patient with a symptomatic left astrocytoma involving the inferior frontal and superior temporal lobes and the insula; transverse, coronal, and sagittal views, 3D surface view. fMRI revealed a left language dominance and a good spatial agreement between the functional localizations obtained with four different language paradigms for both essential language areas (Color figure online)
Additional attention needs to be paid to technique-related inaccuracies due to the superposition of functional and morphological images, the referencing of fMRI data in neuronavigation systems, or the surgery-induced localization errors following removal of brain tissue or loss of cerebrospinal fluid (brain shift) (Reinges et al. 2004; Wittek et al. 2005). Safety of surgical procedures in functional areas or their proximity can be improved by concomitant visualization of functional landmarks and important fiber tracts (e.g., pyramidal tract, arcuate fascicle) using diffusion tensor imaging (DTI) (Coenen et al. 2001; Holodny et al. 2001; Krings et al. 2001; Stippich et al. 2003a; Ulmer et al. 2004b; Holodny et al. 2005; Shinoura et al. 2005). The same holds true for the combined use of fMRI and EEG (Towle et al. 2003), MEG (Kober et al. 2001; Grummich et al. 2006) or PET (Baumann et al. 1995; Bittar et al. 1999). Tumor-induced hemodynamic changes can lead to erroneous fMRI localization and missing or artificial BOLD signals (Holodny et al. 1999, 2000; Schreiber et al. 2000; Krings et al. 2002; Ulmer et al. 2004a, b). Electrophysiological techniques are unsusceptible to these problems, since they directly measure electromagnetic fields resulting from synaptic activity (Berger 1929; Hari and Ilmoniemi 1986; Hämäläinen et al. 1993). However, localization of electromagnetic sources requires complicated modeling and calculations and is therefore limited in precision and accuracy. Furthermore, it should be borne in mind that fMRI and DTI results are generated by using mathematical correlations and therefore vary with the chosen statistical parameters or thresholds. Consequently, the size and extent of fMRI cortical activations or of reconstructed DTI fiber tracts do not reflect the truth and may confront the operator with and unfounded safety margin. Thus, resection borders cannot be determined reliably on the basis of fMRI or DTI data without standardisation and validation.
When applied in a standardized way, fMRI has the diagnostic potential to substantially contribute to the planning and implementation of function-preserving therapies in patients with brain tumors and epilepsy (Lee et al. 1999; Gaillard et al. 2000; Grabowski 2000; Hirsch et al. 2000; Sunaert and Yousry 2001; Baxendale 2002; Binder et al. 2002; Rutten et al. 2002; Stippich et al. 2003a, b; Van Westen et al. 2005; Stippich et al. 2007; Thulborn 2006). Prior to and during neurosurgical interventions, fMRI can help to reduce the need for invasive diagnostic procedures, as intra-arterial WADA test (Wada and Rasmussen 1960; Rausch et al. 1993; Benbadis et al. 1998; Abou-khalil and Schlaggar 2002) or intraoperative electrocorticography (Penfield 1937; Cedzich et al. 1996; Duffau et al. 2002, 2003). Whether the embedding of fMRI and DTI in presurgical diagnostics can indeed reduce surgery-related morbidity and mortality should be investigated in prospective clinical trials (Zacharaki et al. 2012). Prerequisites are a consensus on measuring techniques, analysis procedures, and medical evaluation of clinical fMRI as well as the establishment of official recommendations and guidelines by the assigned medical association. As stated earlier, first attempts in that direction are underway.
5 Content Overview
Chapter 2 provides an easy, but in-depth, access to the techniques and underlying physiology of the abovementioned different fMRI and DTI techniques (Bandettini et al. 1992; Kwong et al. 1992; Ogawa et al. 1993; Basser et al. 1994; Purdon and Weisskoff 1998). The principles and basics of MRI, fMRI, and DTI, as well as the different established experimental designs for task-based fMRI, are presented along with the options for specific data analysis. The resting-state fMRI technique is explained in detail and what methods can be implemented to study functional connectivity in different resting-state networks. Finally, diffusion tensor imaging and tractography are addressed (LeBihan and Johansen-Berg 2012). On a solid methodological basis, the capabilities to study structural connectivity and to employ presurgical tractography are demonstrated.
Chapter 3 extensively deals with the relevant morphological and functional neuroanatomy to facilitate the reader’s access to presurgical fMRI and DTI. In addition to a detailed systematic description of the surface anatomy and cytoarchitecture of the human brain, important morphological criteria for a straightforward identification of different anatomical structures are discussed. Comprehensive information on the functional neuroanatomy of the motor and language systems is provided. Here, it cannot be sufficiently stressed that a profound knowledge of the relevant neuroanatomy is indispensable for a correct medical interpretation of clinical fMRI and DTI data. In any case, the precentral knob is the only reliable anatomical landmark for a functional area, namely, the motor hand representation (Yousry et al. 1997; Fesl et al. 2003). There are no morphological landmarks for areas related to cognitive brain functions such as language or memory (Ojemann et al. 1989; Ojemann 1991). Due to the vast physiological interindividual anatomical variability (Amunts et al. 1999, 2000) and the dependence on various individual factors, functional areas are traditionally mapped intraoperatively by means of electrophysiological methods (Penfield 1937, 1950; Woolsey et al. 1979; Ojemann et al. 1989; Ojemann 1991; Uematsu et al. 1992; Cedzich et al. 1996; Duffau et al. 1999). However, this information is preoperatively inaccessible, and the time needed for surgery is therefore significantly prolonged. FMRI and DTI, on the other hand, yield relevant diagnostic information on anatomy, pathology, and function prior to surgery in one single examination. Thus, the indication for presurgical fMRI and DTI results from:
Clinical signs and symptoms
The limitations of morphological imaging
The necessity to measure and visualize normal, modified neuroplastic, and pathological (e.g., epileptic) brain activity
Chapter 4 covers presurgical task-based BOLD fMRI, which represents fMRI’s best established, validated, and most widely used clinical application. Particular notice is given to practical and methodological-technical aspects (Sect. 3) as well as to specific requirements of fMRI diagnostics in patients with rolandic brain tumors (Sect. 4) or lesions with critical spatial relationship to language areas (Sect. 5). Diagnostic aims and selection criteria for patients are discussed, clinically tested examination protocols are proposed (Stippich et al. 1999, 2000, 2002b, 2003b, 2004, 2005, 2007; Stippich 2010), and their applications are illustrated with example cases. Further, potentials and limits of presurgical task-based fMRI are depicted (Sect. 6).
Chapter 5 highlights the initial experience with resting-state fMRI (RS-fMRI) as a novel functional neuroimaging modality in the presurgical work-up of patients with brain tumors (Mitchell et al. 2013). Here, no stimulation or active cooperation of the patients is required to assess important functional systems, which is potentially of great value to further facilitate the clinical application of fMRI (Posse et al 2013). RS-fMRI provides additional information on the connectivity between different functionally important brain areas, which may be exploited in the future to study the effects of lesions, treatment, and medication on brain function (Niu et al 2014; Smucny et al. 2014; Otten et al. 2012; Martino et al. 2011). Such neuroplastic alterations in brain function may be compensatory or pathological in nature.
Chapter 6 refers to the highly specific application of presurgical fMRI in patients with epilepsy which requires fundamentally different data acquisition and processing techniques as compared to both task-based and resting-state fMRI, such as EEG-correlated fMRI for the localization of epileptogenic foci (Centeno and Carmichael 2014: Chiang et al. 2014). To this end, major technical and methodological problems had to be solved, which now enables us to use electrical devices (EEG) inside MR scanners to correct for interferences and related artifacts and thereby precisely detect and localize the sources of epileptic activity in the human brain. EEG-correlated fMRI is by no means a standard application (Pittau et al. 2014). It should be considered as a valuable additional investigation tool in the field of epilepsy imaging which is available in some leading medical centers worldwide.
Chapter 7 accounts for DTI and DTT with their proven value for functional brain tumor surgery with respect to both preoperative neuroimaging and intraoperative navigation (Potgieser et al. 2014; Shahar et al. 2014; Sternberg et al. 2014; Kuhnt et al. 2013a, b). In this setting, DTI is fully complementary to fMRI, by providing additional relevant information on the course and integrity of important white matter tracts in relation to a surgical target. Hence, these techniques should be applied in combination whenever possible. Specific attention is given to the corticospinal tract and sensorimotor system; the arcuate, uncinate, inferior fronto-occipital fascicles and language systems; as well as the superior longitudinal fascicle, optic radiation, and the visuospatial attention network.
Chapter 8 addresses the specific aspects of functional neuronavigation (Orringer et al. 2012; Risholm et al. 2011). With the help of the functional landmarks provided by fMRI and DTI, the spatial relationship between the brain tumor or epileptogenic zone, functional brain areas, and essential white matter tracts can be evaluated preoperatively, which facilitates the selection of the most cautious therapy. Functional neuronavigation permits planning and implementation of radical and, at the same time, function-preserving operations. This goal is rarely achieved with morphological information alone, especially in the presence of brain malformations, anatomical variants, or a disturbed or destroyed anatomy due to tumor growth or pathology-related neuroplastic changes of brain function. Technical inaccuracies and corrections for brain shift due to intraoperative loss of cerebrospinal fluid and tissue removal have to be taken into account (Kuhnt et al. 2012a).
Chapter 9 is dedicated to the validation of presurgical fMRI and DTI with established reference procedures such as intraoperative electrocorticography (ECoG) or intra-arterial administration of barbiturates (Wada test) (Leclercq et al. 2011). For a thorough understanding of the topic, these reference procedures are presented in detail together with a repetition of the relevant methodological aspects and limitations of fMRI. Today, it can be assumed that presurgical task-based fMRI is a robust and valid tool to localize different representations of the human body in the primary motor and somatosensory cortices, to localize essential language centers – namely, Broca’s and Wernicke’s areas – and to lateralize the brain’s dominant hemisphere for language function. There is also substantial evidence that DTT is reliable to visualize the pyramidal tract, the arcuate fascicle, the optic radiation, and other large white matter bundles both pre- and intraoperatively (Shahar et al. 2014). FMRI and DTI have the potential to help to reduce the number of invasive measures needed, to better select those patients who require such interventions, and to facilitate the planning and targeted intraoperative positioning of electrodes. If and to what extent a substitution of invasive measures may be appropriate is not fully clear, yet.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree