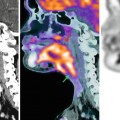
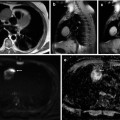
Fig. 35.1
Squamous cell carcinoma of the cervix in a 79-year-old female. T2-weighted transverse (a), sagittal (b), and coronal (c) images demonstrate a large cervical mass with irregular margins. Parametrial extension is seen bilaterally (a). Small bowel involvement is seen in b (arrow) indicating the utility of multiplanar orthogonal views
35.2.2.2 Dynamic Contrast-Enhanced MRI (DCE)
This functional imaging technique gives an assessment of tumour vascularity. Consecutive, three-dimensional, fat-suppressed gradient echo images through the organ of interest are acquired rapidly before, during and after a bolus injection of a paramagnetic contrast agent, such as gadolinium, that increases T1 signal intensity. The degree of enhancement in the early vascular phase is determined by blood flow, vascular density, capillary permeability and capillary surface area and in the interstitial phase by extravascular space volume. The pharmacokinetic profile of the injected contrast agent allows generation of quantitative parameters such as the transfer constant K trans and extracellular extravascular leakage space v e [3]. The DCE data can also be analysed semiquantitatively, producing parameters such as IAUGC (initial area under the gadolinium curve). The uterine tumour enhances poorly and the peak enhancement of the highly perfused myometrium occurs at 90 s post-contrast administration. Therefore, an optimum imaging time is between 60 and 90 s when contrast difference between the uterine tumour and the myometrium is maximal. Cervical mucosa starts to enhance at approximately 30 s post-contrast administration and peaks at approximately 120 s. The fibromuscular stroma of the cervix enhances more slowly [2].
35.2.2.3 Diffusion-Weighted Imaging (DWI)
Diffusion-weighted imaging (DWI) exploits the thermally driven motion of water molecules, which in biological tissue is modified by cell membrane integrity, extracellular microarchitecture, active transport mechanisms and microcirculation and detects molecular displacements at a cellular scale. No exogenous contrast agent is necessary because the image contrast is derived from the differences in the restriction of water movement between the tissues. Fat saturation is routinely applied to diffusion-weighted images. Alterations in the amplitude, duration and spacing of magnetic field gradients can incrementally sensitise DWI to diffusion. The diffusion weighting is determined by the b value, with values from 0 to 2,000 smm−2 currently used in clinical practice. At relatively low b values (<100–150 smm−2), rapid signal loss occurs in structures where water motion is relatively free, for example, in fluid collections, flowing vessels or ducts. This phenomenon is termed intravoxel incoherent motion (IVIM) and gives a perfusional component to the diffusion data. The quantitative parameter apparent diffusion coefficient (ADC) is derived from the exponential attenuation of signal intensity between at least two b values. Restricted diffusion is indicated by bright foci on the high b value diffusion images, with a corresponding area of low signal on the ADC map (Fig. 35.2). It is important to compare the DWI with the corresponding morphological imaging to improve anatomical correlation, as DWI has low signal-to-noise ratio, resulting in poor spatial resolution (Fig. 35.2).
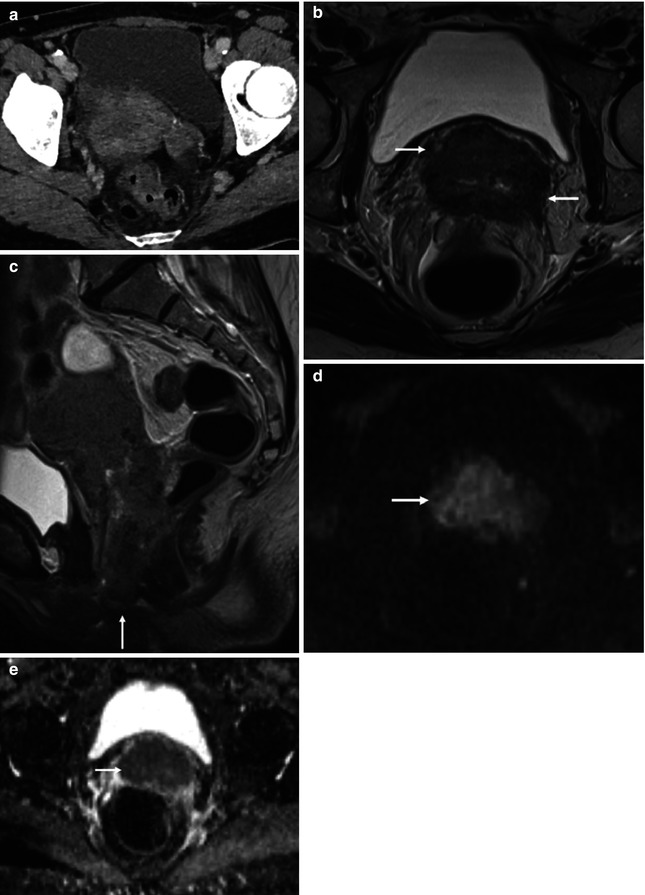

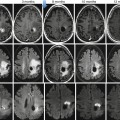
Fig. 35.2
Squamous cell carcinoma of the cervix in a 70-year-old female: transverse CT (a), T2-weighted transverse (b), and sagittal (c) images demonstrate a bulky mass centered on the cervix and extending into both parametria (b, arrows) and inferiorly to the vaginal introitus (c, arrow). Tumor involves the adjacent anterior rectal wall (c). The tumor (d and e, arrow) demonstrates impeded diffusion on the transverse DWI (d, b = 1,200 smm−2) and corresponding ADC map (e, b = 50, 400, 800, 1,200 smm−2)
35.2.2.4 Intrinsic Susceptibility-Weighted (ISW) or Blood Oxygen-Level-Dependent (BOLD) MRI
Intrinsic susceptibility-weighted (ISW) or blood oxygen-level-dependent (BOLD) MRI images are typically a T2* gradient echo sequence. Deoxyhaemoglobin is paramagnetic and causes a slight increase in T2*. ISW/BOLD MRI uses this property to reflect the oxygenation status of tissue immediately adjacent to perfused microvessels. Unfortunately, T2* readings can also be affected by blood flow, pH, carbon dioxide tension and haematocrit. The BOLD effect can be quantified by plotting the natural log of signal intensity on the T2* sequence against the echo time to derive tissue relaxivity. The quantitative parameter, R2*, is derived from the power of the exponent (1/T2*). When the blood volume in the tissue of interest is known, these R2* maps reflect the local concentration of deoxyhaemoglobin. The change in signal intensity in ISW/BOLD is low, and therefore, the R2* gives an assessment of differential areas of oxygenation. The use of vasomodulation techniques with BOLD/ISW MRI, such as breathing carbogen (5 % CO2, 95 % O2) or 100 % oxygen, enables robust measurements or direct correlation with pO2 values. These vasomodulation techniques augment the ISW/BOLD signal by increasing perfusion as a result of CO2 vasodilatory effects and increased oxygenation. These vasomodulation techniques are not well tolerated in human studies due to technical difficulties in gas delivery and patient distress. In a preliminary study by Hallac et al. [4], BOLD/ISW MRI at 3 T was feasible in cervical cancer with all 10 patients and 3 healthy volunteers tolerating the 100 % oxygen vasomodulation technique.
35.2.2.5 Proton Magnetic Resonance Spectroscopy (MRS)
Proton magnetic resonance spectroscopy (MRS) derives its signal from the different resonant frequencies of protons within a molecule due to magnetic diatomic interactions within the molecular structure. Data may be acquired from a single voxel of interest using Point-REsolved SpectroScopy (PRESS) or a STimulated Echo Acquisition Mode (STEAM). A CHEmical-Shift Selective (CHESS) pulse is commonly used for water suppression. The acquisition is fairly fast (1–3 min). Two-dimensional or three-dimensional chemical shift imaging obtains data from several voxels within a slab or volume of tissue by repetition of PRESS- or STEAM-type sequences to which spatial phase encoding has been added. Acquisition time relates to the number of phase-encoding steps and is in the order of 10–20 min. Resonant signals are displayed as a function of frequency (spectrum) after Fourier transform as a series of peaks on the chemical shift axis (expressed in parts per million, ppm), which are unique for different metabolites. The ppm scale describes the shift in hertz from a reference frequency divided by the excitation frequency of the external magnetic field. Although spectral resolution is improved at higher magnetic field strengths (3 T and above) due to the higher signal-to-noise ratio, inherent field inhomogeneities result in metabolites resonating at a range of frequencies; nevertheless, typical peak patterns can be recognised (choline 3.2 ppm, creatine 3.0 ppm, lactate 1.4 ppm). Absolute quantification in vivo is hampered by the absence of internal standards of concentration so relative concentrations are frequently used to characterise tumour and assess response.
35.3 Staging
35.3.1 Local Staging
35.3.1.1 Endometrium
Endometrium T2-W imaging in two or three planes orthogonal to the uterine body is the mainstay of detection and staging endometrial cancer. The revised International Federation of Gynecology and Obstetrics (FIGO) staging system is given in Table 35.1 [5]. The depth of myometrial invasion by endometrial carcinoma on preoperative imaging assessment is an important distinction as it strongly affects the incidence of metastasis to regional nodes and may therefore influence surgical strategies (Figs. 35.3 and 35.4).
Stage Ia | Tumour confined to the corpus uteri |
IAa | No or less than half myometrial invasion |
IBa | Invasion equal to or more than half of the myometrium |
Stage IIa | Tumour invades cervical stroma, but does not extend beyond the uterusb |
Stage IIIa | Local and/or regional spread of the tumour |
IIIAa | Tumour invades the serosa of the corpus uteri and/or adnexac |
IIIBa | Vaginal and/or parametrial involvementc |
IIICa | Metastases to pelvic and/or para-aortic lymph nodesc |
IIIC1a | Positive pelvic nodes |
IIIC2a | Positive para-aortic lymph nodes with or without positive pelvic lymph nodes |
Stage IVa | Tumour invades bladder and/or bowel mucosa and/or distant metastases |
IVAa | Tumour invasion of bladder and/or bowel mucosa |
IVBa | Distant metastases, including intra-abdominal metastases and/or inguinal lymph nodes |

Fig. 35.3
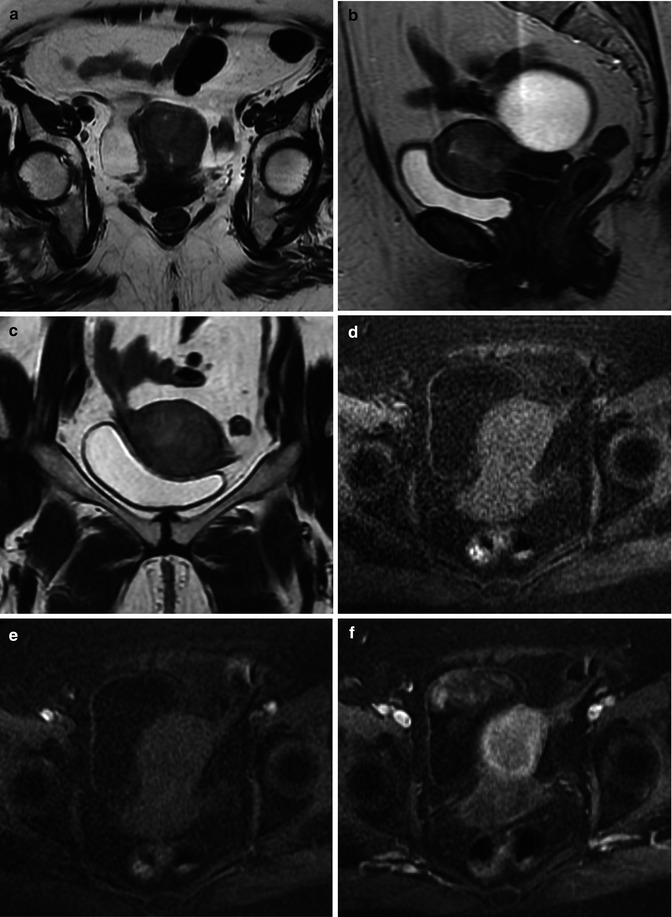
Endometrial cancer in a 58-year-old female: sagittal T2-weighted image (a) demonstrates a large intermediate signal endometrial mass with myometrial invasion superiorly (arrow) and cervical stromal involvement at the internal os (dotted arrow). The normal myometrium shows progressive enhancement on the axial T1-weighted volumetric interpolated breathhold examination (VIBE) fat saturated series; [pre- (b), immediate (c), 1 min (d), and 2 min (e) post- gadolinium], compared to the endometrial tumor, which enhances poorly. The contrast between tumor and myometrium is maximal at 2 min post-gadolinium, making the myometrial invasion identifiable as superficial only (<50 % myometrial involvement) (arrows, e)


Fig. 35.4
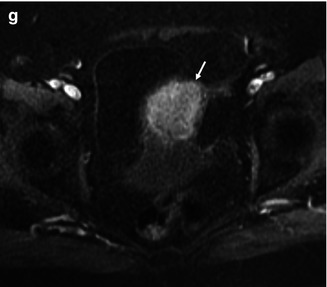
Endometrioid adenocarcinoma of the endometrium in a 80-year-old female. T2-weighted transverse (a), sagittal (b), and coronal (c) demonstrate abnormal intermediate signal intensity affecting the uterine corpus, in keeping with a diffuse infiltrative tumor. Transverse T1-weighted VIBE fat saturated images [pre- (d), immediate (e), 1 min (f), and 2 min post- gadolinium (g)] demonstrate a poorly enhancing endometrial tumor with deep myometrial invasion (arrow)
MRI using a T2-W sequence best demonstrates disruption of normal myometrial zonal differentiation. In the postmenopausal patient, uterine involution and reduction of zonal differentiation make it more difficult to assess myometrial infiltration. Some early studies advocated the addition of contrast enhancement because of a higher demonstrable accuracy for delineating the tumour than endovaginal ultrasound or CT [6, 7], although more recently this has been superseded by higher resolution T2-weighted imaging as a result of improvements in scanner hardware and software. MRI at 3 T does not offer significant benefit over 1.5 T, although faster imaging protocols may be helpful in uncooperative patients.
Gadolinium-enhanced dynamic sequences increase the accuracy of MR imaging in diagnosing the depth of myometrial invasion (Figs. 35.3 and 35.4). In particular, they improve the visualisation of the inner myometrium, the so-called subendometrial enhancing zone, whose disruption or changes are essential for diagnosing myometrial invasion. The major diagnostic advantage of these enhanced techniques is in postmenopausal women, where visualisation of the junctional zone may be difficult on the T2-W sequences. In postmenopausal women, the accuracy in estimating myometrial invasion with T2-W images, contrast-enhanced T1-weighted images and dynamic contrast-enhanced MRI was 66.7, 77.8 and 92.6 %, respectively [8]. In a more recent study of 45 women, Nasi et al. [9] showed that gadolinium enhancement with Fast MultiPlanar Spoiled GRadient echo (FMPSPGR) had a global sensitivity and specificity of 90.6 and 93.3 %, respectively, with a mean negative predictive value of 96.3 % and a mean positive predictive value of 88 % compared to the FSE T2-W sequence (global sensitivity and specificity of 80.6 and 87.6 %, respectively, mean negative predictive value 92.6 %, mean positive predictive value 86 %) giving contrast-enhanced sequences a higher staging accuracy (95 % vs.78 %).
Although DCE is routinely used for staging endometrial cancer and is highly accurate, DWI also can be useful in determining the depth of myometrial invasion. It can be particularly helpful in tumours that are either iso- or hyperintense relative to the myometrium on T2-W images or when the use of intravenous contrast medium is contraindicated. Recently, in a prospective study, Rechichi et al. 2010 [10] suggested that the accuracy of DW MRI in assessing myometrial invasion was comparable to DCE MRI which it could replace in preoperative evaluation.
DWI is also useful in detecting the presence of endometrial cancer. On DW images, endometrial cancer demonstrates high signal intensity with corresponding low signal intensity on ADC maps: the quantified ADC values were discriminatory between benign and malignant endometrial lesions [11]. The mean ± SD ADC of stage IA endometrial carcinoma has been reported as 0.878 ± 0.185 × 10−3 mm2/s, which was significantly lower (P < 0.01) than that of normal endometrium (1.446 ± 0.246 × 10−3 mm2/s) and benign endometrial lesions (1.637 ± 0.178 × 10−3 mm2/s) without any overlap [12]. In a study of 25 patients, sensitivity, specificity and accuracy for detecting tumour were 84.6, 100 and 92 %, respectively [13]. However, one should be aware that there is no reliable cut-off ADC value that is diagnostic of presence of malignancy. DWI can also be helpful in cases where the endometrial biopsy is technically impossible, the histopathology results are inconclusive or when the MRI is performed for different clinical indications, such as evaluation of an indeterminate adnexal lesion, and the endometrial abnormality is an incidental finding.
High-grade endometrial carcinomas have high cellular density and may be expected to have lower ADC values compared to low-grade tumours. However, ADC values do not correlate with histological tumour grade, the depth of myometrial invasion or whether lymph node metastases are present [14]. The hope that DWI may influence surgical planning by improving preoperative detection of high-grade tumours therefore is not a clinical reality; this may in part be due to the tumour necrosis associated with poorly differentiated tumours that can increase ADC values.
DW MRI can be also useful in incidental detection of drop metastases within the cervix or metastatic foci outside the uterus, such as adnexa or peritoneum [15].
35.3.1.2 Cervix
The primary tumour is best assessed using T2-weighted MR imaging which is superior to CT in detection (sensitivity 75 % vs. 51 %, p < 0.005) and in staging (accuracy 75–77 % vs. 32–69 %, p <0.025) [16, 17]. The revised FIGO staging is given in Table 35.2 [5]. The soft tissue contrast of MR imaging differentiates intermediate signal intensity tumour from low signal intensity stroma and identifies the presence and extent of the tumour in three planes orthogonal to the cervix. The coronal and axial planes are used for determining direct extension into the parametrium, the axial plane for determining extension into the bladder and rectum and the sagittal plane for determining extension into the uterine body, bladder and rectum [18]. Fast spin-echo T2-W images provide the best image contrast. Measuring tumour volume on MRI is of paramount importance, the size of tumour burden is a stronger predictor of outcome than invasion beyond the anatomical margins of the uterus [19], and it also gives an indication of likely lymph node involvement [20]. MRI has been found to be accurate in assessing cervical tumour dimensions to within 0.5 cm [21–26], and tumour volume at MRI compared to histology has shown good correlation (up to 0.98) [21, 27–30], but MRI volumes correlate only weakly with clinical stage [31].
Stage I | The carcinoma is strictly confined to the cervix (extension to the corpus would be disregarded) |
IA | Invasive carcinoma, which can be diagnosed only by microscopy, with deepest invasion ≤5 mm and largest extension ≤7 mm |
IA1 | Measured stromal invasion of ≤3.0 mm in depth and extension of ≤7.0 mm |
IA2 | Measured stromal invasion of >3.0 mm and not >5.0 mm with an extension of not >7.0 mm |
IB | Clinically visible lesions limited to the cervix uteri or preclinical cancers greater than stage IAa |
IB1 | Clinically visible lesion ≤4.0 cm in greatest dimension |
IB2 | Clinically visible lesion >4.0 cm in greatest dimension |
Stage II | Cervical carcinoma invades beyond the uterus but not to the pelvic wall or to the lower third of the vagina |
IIA | Without parametrial invasion |
IIA1 | Clinically visible lesion ≤4.0 cm in greatest dimension |
IIA2 | Clinically visible lesion >4.0 cm in greatest dimension |
IIB | With obvious parametrial invasion |
Stage III | The tumour extends to the pelvic wall and/or involves lower third of the vagina and/or causes hydronephrosis or nonfunctioning kidneyb |
IIIA | Tumour involves lower third of the vagina with no extension to the pelvic wall |
IIIB | Extension to the pelvic wall and/or hydronephrosis or nonfunctioning kidney |
Stage IV | The carcinoma has extended beyond the true pelvis or has involved (biopsy proven) the mucosa of the bladder or rectum. A bullous oedema, as such, does not permit a case to be allotted to stage IV |
IVA | Spread of the growth to adjacent organs |
IVB | Spread to distant organs |
Dynamic contrast enhancement was first advocated over a decade and a half ago for evaluating cervical cancer. Over this time, perfusion studies have largely focused on evaluating sensitivity and specificity of stromal invasion. In a correlative study, DCE gave a higher sensitivity and specificity for diagnosing stromal invasion >3 mm than standard T2-weighted imaging (accuracy 98 % vs. 76 %) [32]. Contrast enhancement has been used for assessment of tumour angiogenesis [33] and vascular density has been associated with low pO2 in preliminary validation studies in cervical cancer [34] with a positive correlation observed between level of contrast enhancement on DCE and pO2 measured by oximetry [35]. However, there is no diagnostic advantage of DCE over T2-W imaging in staging cervical cancer and no correlation with tumour aggressiveness [36] so that these images are not routinely used.
With the introduction of screening, the emphasis has shifted towards detecting early-stage disease. In these patients with clinical IA and IB1 cervical cancer referred following cone biopsy on which invasive disease is an unexpected finding, identifying disease within the cervix is difficult on T2-W imaging because it is of small volume. Also, patients are often referred following positive cone biopsies when most or all of the invasive disease has been removed. Image resolution with external phased array coils is limited with small-volume disease and can be significantly improved by the use of receiver coils close to the region of interest as they provide significant increases in signal-to-noise ratio. An endovaginal technique is well tolerated and has been shown to be accurate in the detection and location of cervical cancers and exclusion of early parametrial extension [27, 37–39] and has been found to be more sensitive than external coil techniques in the detection of small-volume disease. Because local oedema and granulation tissue make distinction between residual tumour and post-operative change difficult with an endovaginal technique using T2-weighted images, DWI sequences have been implemented to differentiate small tumours from postsurgical change based on water diffusion within tissues (Fig. 35.5). An improved sensitivity for tumour detection has been found when DWI is used in conjunction with T2-W MRI in these cases with good interobserver agreement [40].
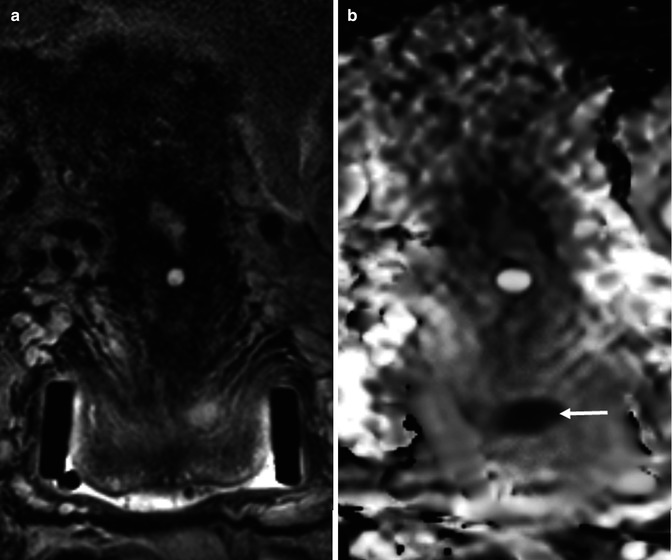

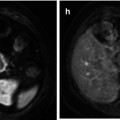
Fig. 35.5
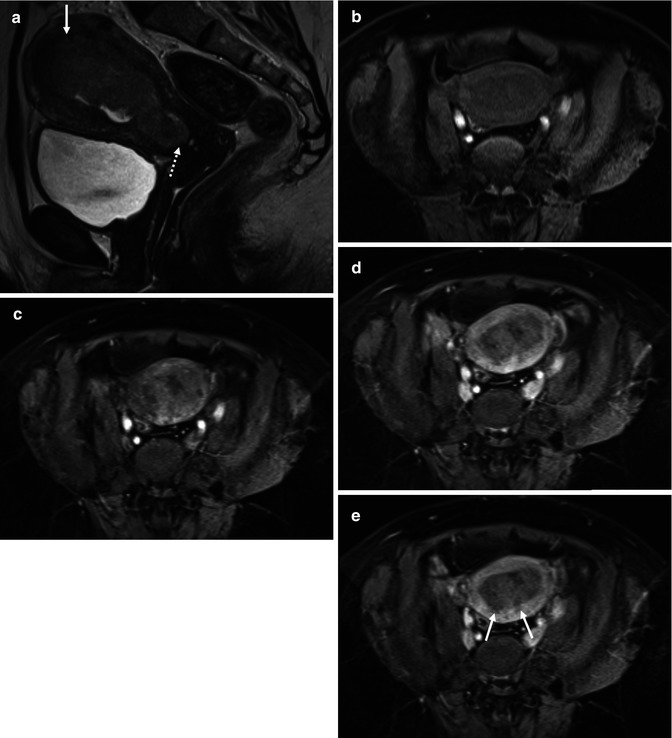
Squamous cell carcinoma of the cervix in a 26-year-old female who is being considered for fertility sparing surgery. Endovaginal coronal T2-weighted image (a) demonstrates a large LLETZ defect with a small and ill-defined area of intermediate signal in the roof of this defect. This represents a small cervical tumor, which is better appreciated on the corresponding ADC map (b), (b = 0,100, 300, 500, 800 smm−2) as a focal area of low signal (arrow)
Malignant cervical tissue demonstrates restricted diffusion and hence reduced ADC values when compared to normal tissue. High b value (>800 s/mm2) DWI and ADC maps allow differentiation of benign from malignant zones of cervix with high sensitivity and specificity [41–45]. Preliminary data also demonstrates the ability of ADC to differentiate histological type and grade of tumour [44, 46]. In a prospective study, ADC values were statistically significantly lower in malignant compared to benign cervical tissue (1.1 × 10−3 mm2/s vs. 1.7 × 10−3 mm2/s, P < 0.001) and poorly differentiated compared to well-differentiated tumours (1.1 × 10−3 mm2/s vs. 1.2 × 10−3 mm2/s, P = 0.01) [44]. However, there was no significant difference when tumours were divided by other histological features (type, lymphovascular invasion and lymph node metastasis). More recently, an increasing literature suggests ADC of squamous carcinomas to be lower than adenocarcinomas [46, 47] but needs quantification rather than visualisation alone for evaluation of these differences.
CT and MRI have both been used to assess parametrial spread: both result in false-positive diagnoses [24, 48] likely from misinterpreting inflammatory parametrial soft tissue strands associated with tumour as actual tumour invasion. DCE is superior to conventional T2-W images and T1-W post-contrast images in detecting the presence of parametrial extension by distinguishing tumour from normal cervical stroma and myometrium [49].
There is no evidence for the benefit of MRS in diagnosis of cervical cancer. In the proton spectrum, changes have largely been reported in the lipid and choline fractions. A doubling of lipids on ex vivo spectroscopy in malignant cervical tissue compared with benign cervical tissue has been reported [50] although there was no correlation with tumour load. Choline observed in vivo in cervical cancer was not significantly different from that reported in benign lesions [51] so that this technique is not yet clinically useful.
35.3.2 Nodal Staging and Distant Spread
The assessment of lymph node status with functional MRI techniques remains poor. In a study of 37 patients with endometrial cancer, sensitivity, specificity, diagnostic accuracy and positive and negative predictive values for lymph node assessment on contrast-enhanced MRI were 50, 95, 90, 50 and 95 %, respectively [52], so that recognition of abnormal lymph nodes is still dependent on size criteria. This prompted endeavour on developing extrinsic contrast agents for detecting metastatic nodes. Over the last decade, there has been significant research activity around production of a lymph node-specific MR contrast agent to allow the identification of malignant nodal infiltration independent of the lymph node size. This novel MR contrast agent is a nanoparticle (mean diameter, 30 nm) and is composed of an iron oxide core, coated with low molecular weight dextran (ultrasmall particles of iron oxide, USPIO). The particles, administered intravenously, are taken up by macrophages in the reticuloendothelial system, predominantly within the lymph nodes. Their uptake results in marked loss of signal intensity (darkening) of the node on T2- and T2*-weighted sequences because of a susceptibility artefact caused by the iron. Metastatic tissue within a node displaces the normal macrophages, thus preventing uptake of contrast agent, and the node continues to remain high in signal intensity. Initial studies indicated a significant increase in sensitivity, with no loss of specificity for the detection of malignant lymph nodes with USPIOs in patients with cervical and endometrial cancer [53]. On a node-by-node basis, the sensitivity increased from 29 % using standard size criteria to 93 % using USPIO criteria. On a patient-by-patient basis, sensitivity increased from 27 % using standard size criteria to 100 % using USPIO criteria. Unfortunately, these data were not confirmed when the agent was trialled in a phase 3 setting and has resulted in a lack of its licensing, so that it is currently unavailable for clinical use.
With the advent of DWI in oncological imaging, this intrinsic contrast mechanism has been explored for improving detectability of nodes containing metastatic deposits. The European Society of Urogenital Radiology states that diffusion-weighted sequences are optional in staging cervical cancer but are recommended to help evaluate lymph nodes and to detect a residual lesion after chemoradiotherapy [54]; however, there is a paucity of evidence to support this. A number of small single centre studies have reported on the potential of DWI in nodal staging: using the minimum ADC criteria (≤0.881 × 10−3 mm2/s), Liu et al. [55] showed that the sensitivity and specificity for differentiating metastatic from non-metastatic lymph nodes were 95.7 and 96.5 %, respectively. However, in a preliminary study of 26 women with cervical cancer who underwent whole body DWI, the mean ADC value of 16 metastatic nodes was much higher (0.96 ± 0.14 × 10−3 mm2/s), although significantly lower than that of benign nodes (1.39 ± 0.19 × 10−3 mm2/s, P = 0.00). In this study, an ADC threshold value of 1.14 × 10−3 mm2/s gave a sensitivity of 83 %, specificity 98 % and accuracy 94 % for detecting lymph node involvement [41]. Another retrospective study with 7 positive lymph nodes also suggested significant differences between ADCs of benign vs. malignant nodes, and an ADC threshold value of 0.807 × 10−3 mm2/s gave a sensitivity, specificity, positive and negative predictive value and accuracy of 100, 98.3, 63.6, 100 and 98.3 %, respectively [56]. However, these studies did not differentiate between small and enlarged nodes, leading to significant reporting bias.
Studies that have included size criteria as well as ADC in their evaluation are more useful. The combination of size and relative ADC values may well be useful in detecting pelvic lymph node metastasis in patients with cervical and uterine cancers (improved sensitivity of 83 % vs. 25 % for the same specificity of 98 % vs. 99 %) with the smallest metastatic lymph node detected being 5 mm in short axis [57]. However, the literature remains conflicting: for example, one study of 680 lymph nodes from 143 patients who underwent radical hysterectomy for uterine cervical cancer gave a specificity and accuracy of mean ADC of 77 and 77 %, compared to 65 and 67 % for short-axis diameter [58], while another prospective study of 18 patients and 340 dissected nodes showed that the differences in the short-axis diameter, the long-axis diameter and the ratio of short- and long-axis diameter on T2-W images between metastatic and non-metastatic nodes and the differences in the ADC values between metastatic and non-metastatic nodes were not significant [59]. A recent small prospective study with good histological correlation, which took into account lymph node size and observer variation, showed that the sensitivity and specificity for detecting lymphatic metastasis by predefined conventional MRI RECIST characteristics were 33 and 83 % on a patient level and 33 and 97 % on regional level, respectively, for observer 1 and 33 and 93 % on a patient level and 25 and 98 % on regional level for observer 2. The kappa value for reproducibility of metastasis detection on regional level was 0.50. The short-axis diameter showed the highest diagnostic accuracy (area under the curve (AUC) = 0.81); ADC did not improve diagnostic accuracy (AUC = 0.83) [60]. No multicenter trial evaluating lymph node status prospectively with ADC as yet exists and is badly needed to address this issue.
35.4 Impact of Optimal Imaging on Clinical Decision Making
35.4.1 Endometrium
Abnormal uterine bleeding in a postmenopausal woman is the most common presenting symptom of endometrial cancer so diagnosis is usually made early, resulting in high survival rates (77 % survival at 5 years) [61]. Following presentation with abnormal uterine bleeding, transvaginal ultrasound is clinically useful in the initial assessment of endometrial cancer and endometrial hyperplasia, with a high sensitivity and specificity of 100 and 79.6 %, respectively [62], identifying those patients with an endometrial thickness of greater than 4 mm who will go on to have an endometrial biopsy. The FIGO classification defines the formal staging of endometrial cancer based on surgical staging and histopathology. Imaging is an important adjunct to clinical and surgical evaluation in the staging and follow-up of endometrial cancer, and MRI is considered to be the most reliable imaging study in this regard [63]. Management of endometrial cancer is by primary surgery, which consists of total abdominal hysterectomy and bilateral salpingo-oophorectomy. Patients with deep myometrial invasion (>50 %) are treated with adjuvant radiotherapy post-operatively to reduce the risk of recurrence. Vaginal brachytherapy is indicated in cases of endocervical involvement. In patients with inoperable disease or those patients who are unable to undergo surgery due to pre-existing comorbidity, imaging is helpful to define tumour extent for palliative treatment with radiotherapy and chemotherapy [64, 65].
Endometrial cancer spread to the regional lymph nodes is an important prognostic factor [66]. A pelvic and para-aortic node dissection is required for full staging, but a recent large randomised trial (ASTEC) demonstrated no benefit in overall or recurrence-free survival of systematic lymphadenectomy [67]. There is a lack of consensus on which women require a lymphadenectomy and the definition of an adequate lymph node dissection [68]. Current practice is for women at high risk of recurrence (based on imaging and biopsy) to undergo pelvic lymphadenectomy at the time of primary surgery. High-risk tumours demonstrate deep (>50 %) myometrial invasion, cervical involvement or poor differentiation compared to low-risk tumours which are well differentiated, with superficial myometrial invasion, and no enlarged lymph nodes on MRI. Chan et al. [69] demonstrated that a more extensive lymphadenectomy improved the 5-year disease-specific survival rate of patients with intermediate- or high-risk endometrioid uterine cancer. Although, MRI is limited in its ability to differentiate between benign and metastatic lymph nodes of similar size [2, 52], preoperative MRI plays a vital role in identifying the intermediate- or high-risk endometrial cancer cases and thus directing surgery. MRI achieves this by predicting depth of myometrial invasion and cervical stromal involvement and identifying enlarged pelvic and para-aortic lymph nodes and distant spread [2, 63]. FDG PET/CT is useful in the preoperative diagnosis of lymph node metastases, with high accuracy and negative predictive values of 96.8 and 97.2 %, respectively [70], but it is not generally considered sensitive enough to replace lymph node dissection [63].
Tumour extension into the cervix imparts a worse prognosis, and its recognition assists treatment planning [52]. Sagittal images provide a longitudinal view of the uterine body and cervix, although it may be difficult to differentiate endometrial tumour from the high-signal endocervical mucosa [2] (Fig. 35.3). Sagittal images are also useful to assess anterior and posterior tumour extension into the urinary bladder and rectum, respectively. For premenopausal patients with endometrial cancer when fertility-sparing surgery is being considered, MRI aids the correct selection of patients by excluding significant myometrial invasion and ovarian pathology, especially in Lynch syndrome [71].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree