▪ Neonatal
Necrotizing Enterocolitis
Necrotizing enterocolitis (NEC) is a disease primarily of premature infants in the intensive care unit. It most often occurs 1 to 3 weeks after birth in infants weighing less than 1000 g but can also less commonly occur in older, nonpremature infants under extreme stress, such as after cardiac surgery. The overall mortality rate is 20% to 30%. NEC is an idiopathic enterocolitis that is most likely related to some combination of infection and ischemia. Symptoms include abdominal distention, feeding intolerance, increased aspirates from nasogastric tube, and sepsis. It is interesting to note that the only parameter associated with decreased incidence of NEC is the use of maternal breast milk. When NEC is suspected, infants are placed in the status of nil per os (NPO), treated with antibiotics, and monitored by serial abdominal radiographs (anteroposterior supine and a free air view [cross-table lateral or left lateral decubitus]).
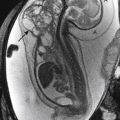
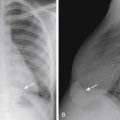
NEC most commonly affects the ileum and right colon. Radiographic findings range from normal to suggestive to diagnostic. Suggestive findings include focal dilatation of bowel (especially within the right lower quadrant) or featureless, unfolded-appearing small bowel loops with separation of the loops, suggesting bowel wall thickening. An unchanging bowel gas pattern over serial films is worrisome. The most definitive finding of NEC is the presence of pneumatosis (gas in the bowel wall) ( Fig. 5-1 ). Pneumatosis appears as multiple bubblelike or curvilinear lucencies overlying the bowel. Its appearance can be similar to that of stool. However, stool is uncommon in sick premature neonates in the intensive care unit. Portal venous gas can also occur (see Fig. 5-1 ). This appears as branching linear lucencies overlying the liver. Free intraperitoneal air is the only radiographic finding seen in NEC that is considered an absolute indication for surgery. Free air may be seen as triangles of anterior lucency on cross-table laterally positioned radiographs ( Fig. 5-2 ); as overall increased lucency on supine-positioned radiographs (see Fig. 5-2 ); in visualization of both sides of the bowel wall (Rigler sign); or as outlining the intraperitoneal structures, such as the falciform ligament (football sign). In the absence of free air, the decision to perform surgery is made by using a combination of clinical and radiographic findings.


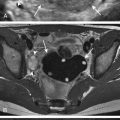
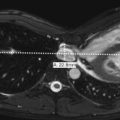
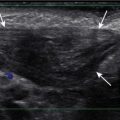
Ultrasound has also been advocated as a means of monitoring infant for NEC ( Figs. 5-3 through 5-6 ) and is used primarily instead of radiography to monitor NEC in some places. In cases of NEC in which the abdomen is distended but relatively gasless, ultrasound is particularly helpful. The identification of thickened bowel loops with increased or absent abnormal degrees of color Doppler flow is suggestive of inflamed (increased flow) or infarcted (decreased flow) bowel. A large amount of free fluid is also a poor prognostic finding. Ultrasound depicts pneumatosis as areas of hyperechoic foci in the bowel wall (see Figs. 5-3 and 5-4 ). In contrast to intraluminal gas, gas related to pneumatosis does not change position with changes in position of the patient. Portal venous gas is also easily depicted by ultrasound as hyperechogenic foci moving within the portal vein (see Fig. 5-5 ). Free air can also be depicted by ultrasound as areas of hyperechogenicity.




A delayed complication seen in survivors of NEC is bowel stricture ( Fig. 5-7 ). These strictures most commonly involve the left colon.

High Intestinal Obstruction in Neonates
Neonates with suspected intestinal obstruction can be divided into those with upper gastrointestinal obstruction and those with lower intestinal obstruction on the basis of clinical symptoms and radiographic findings. Infants with high intestinal obstruction present predominantly with vomiting. Radiographs may show distension involving the stomach, duodenum, jejunum, or all three, depending on the level of the obstruction. The number of distended small bowel loops is much fewer than that seen with distal bowel obstruction. The most common causes of upper gastrointestinal tract obstruction in neonates include duodenal atresia or stenosis, duodenal web, annular pancreas, midgut volvulus or obstruction by Ladd bands, and jejunal atresia ( Table 5-1 ).
Duodenal Atresia, Stenosis, Web, and Annular Pancreas
Duodenal atresia, stenosis, web, and annular pancreas are all part of a spectrum of similar abnormalities. All cause either complete or partial duodenal obstruction and usually present at birth or within the first few days of life. Often, components of more than one diagnosis are present. For example, many cases of duodenal atresia have a component of annular pancreas, and annular pancreas almost never occurs without a component of intrinsic duodenal stenosis.
The duodenum is the most common site of intestinal atresia. Duodenal atresia and stenosis almost always occur in the region of the ampulla of Vater. Approximately 30% of cases of duodenal atresia are associated with Down syndrome. Other associations with duodenal atresia include other intestinal atresias, biliary abnormalities, congenital heart disease, and associations with the complex known as VATER (vertebral defects, imperforate anus, tracheoesophageal fistula, and radial and renal dysplasia). Radiographs of neonates with duodenal atresia typically demonstrate a dilatated stomach and dilatated proximal duodenum with no gas distal to the proximal duodenum. The two dilatated structures are referred to as a “double bubble” sign ( Fig. 5-8 ). In the appropriate clinical setting, a double bubble is diagnostic of duodenal atresia, and additional imaging by an upper gastrointestinal series (UGI) is unnecessary. The question that often arises is how does one know that this is not an acute obstruction caused by a midgut volvulus, which is a surgical emergency? The answer is that dilatation of the duodenal bulb is seen only with chronic causes of obstruction. There is not enough time for the bulb to become dilated in acute obstruction, such as with an acute midgut volvulus. If it is not clear, a UGI can be performed to document the cause of obstruction. With duodenal stenosis, the double bubble is seen in association with the presence of distal bowel gas.

Duodenal web is another cause of congenital duodenal obstruction. Typically a web consists of an obstructing membrane; a pin-sized hole in its center is the only lumen. The web may stretch downstream, forming a windsock configuration seen on a UGI ( Fig. 5-9 ). Because the obstruction is not complete, these patients may present later in life than those with atresia. The presence or absence of a component of annular pancreas is not something that can be determined on a UGI in the setting of congenital duodenal obstruction.

Malrotation and Midgut Volvulus
Midgut volvulus is one of the few true emergencies in pediatric gastrointestinal imaging. A delay in the diagnosis of midgut volvulus can result in ischemic necrosis of large portions of the bowel and possibly death. An understanding of midgut embryogenesis is often emphasized, but an understanding of the end result is more important. With normal embryonic rotation, both the duodenojejunal and ileocolic portions of the bowel rotate 270 degrees about the axis of the superior mesenteric artery (SMA). The result is that the duodenojejunal junction (DJJ) is positioned in the left upper quadrant and the cecum is positioned in the right lower quadrant. This results in a long, fixed base that keeps the small bowel mesentery from twisting. If the duodenojejunal and ileocecal junctions are not in their normal positions, the base of the small bowel mesentery may be short and predispose the small bowel to twisting, resulting in a midgut volvulus.
For clarification, note the following definitions:
- •
Malrotation: Embryologic abnormality of rotation that results in abnormal fixation of the small bowel mesentery that results in a short mesenteric base.
- •
Midgut volvulus: Abnormal twisting of the small bowel around the axis of the SMA. Volvulus can result in bowel obstruction, ischemia, or infarction but is not defined by the presence or absence of obstruction or ischemia.
- •
Ligament of Treitz: Also referred to as the DJJ. This is the anatomic location where the duodenum passes through the transverse mesocolon and becomes jejunum. It is also where the bowel changes from retroperitoneal (duodenal) to intraperitoneal (jejunum). This anatomic location is not visualized but is inferred on imaging.
- •
Ladd bands: Abnormal fibrous peritoneal bands that can occur in patients with malrotation; they are potential causes of duodenal obstruction, in addition to volvulus.
The diagnosis of malrotation has traditionally been made on a UGI by determining that the DJJ is abnormally positioned. The DJJ, the point at which the proximal bowel turns inferiorly on a frontal view, is considered normal when it meets the following two criteria—(1) right to left : it is to the left of the spine and (2) superior to inferior : it is at the same level as or superior to the duodenal bulb. In addition, on the lateral view, the duodenum normally moves posteriorly and then inferiorly before moving back anteriorly. The “inferior” moving position on the lateral view is the retroperitoneal portion. If on the lateral view the duodenum moves posteriorly and then back anteriorly without the portion moving from superior to inferior (the retroperitoneal part), it is suspicious for malrotation. It is important to evaluate the position of the DJJ during the first pass of contrast through the duodenum and jejunum (see subsequent material; Figs. 5-10 and 5-11 ).


In many cases of malrotation the findings are grossly obvious; the duodenum courses rightward, rather than leftward and never crosses the spine ( Fig. 5-12 and see Fig. 5-11 ). However, when performing UGIs in children, there are many cases that do not quite meet the criteria for normal but are very close. It is probably inappropriate to send all of these cases to the operating room. As one becomes more experienced in imaging, some things become more and more clear. Others become less clear, and one realizes that many of the rules one was taught should really be thought of more as guidelines. Although germane to the fabric of pediatric radiology, the diagnosis and exclusion of malrotation are not straightforward or easy. In cases with borderline-positioned DJJs (not quite as high as the bulb, not quite to the left of the spine), most pediatric radiologists follow the contrast through the small bowel (see Fig. 5-11 ). If the jejunum is in the left upper quadrant and the ileum and cecum are in the right lower quadrant, the patient is probably not at risk for midgut volvulus. Furthermore, the DJJ is a mobile structure in children and can “factitiously” be moved into an abnormal position by a space-occupying lesion, such as a mass or distended bowel loops. In addition, the presence of a nasojejunal tube may alter the apparent position of the DJJ.

In patients who are malrotated, midgut volvulus may happen at any age; however, more than 90% are present during first 3 months of life. Midgut volvulus can be seen on a UGI as a corkscrew appearance of the duodenum and proximal jejunum or as duodenal obstruction ( Fig. 5-13 ). The presence of bilious vomiting and findings of malrotation on a UGI, with or without findings of midgut volvulus, is considered a surgical emergency.

Malrotation and midgut volvulus may also be encountered on cross-sectional imaging studies, such as computed tomography (CT) or ultrasound, when these studies are ordered to evaluate abdominal pain or vomiting. This is particularly true in older children in whom malrotation is often not initially suspected as the cause of acute abdominal symptoms. On cross-sectional imaging the bowel may be seen to form a swirling pattern around the superior mesenteric vessels ( Fig. 5-14 ).

Today, there are an increasing number of people advocating ultrasound as the primary modality for infants with bilious vomiting, mainly because of the previously mentioned difficulties in using a UGI to make the diagnosis of malrotation. If an ultrasound shows a swirling pattern of bowel and vessels about the SMA, this is diagnostic of midgut volvulus. Such a child can go straight to the operating room without documenting the finding by a UGI.
Whether ultrasound can also accurately make or exclude the diagnosis of malrotation without volvulus has been more controversial. Initially, a reversal of the normal relationship between the SMA and superior mesenteric vein (SMV) was advocated as a finding of malrotation (see Fig. 5-11 ). Normally the SMV is to the right of the SMA (the SMA is smaller and rounder and surrounded by echogenic fat). However, this has been shown to be neither sensitive nor specific. One study showed that the SMA-SMV relationship was normal in 29% of malrotated patients and only 79% of normal controls. However, more recently, it has also been advocated that if ultrasound shows the third part of the duodenum traversing left to right between the aorta and the SMA, documenting that the duodenum travels retroperitoneal, this excludes malrotation. Conversely, if the duodenum does not traverse between the aorta and the SMA ( Fig. 5-15 ), the child is malrotated. A study has shown that a normal retroperitoneal location of the third portion of the duodenum was seen in 100% of controls and only 2.5% (1 out of 38) of children with malrotation. This suggests this finding is fairly accurate at confirming and excluding malrotation in neonates.

Performing an Upper Gastrointestinal Series in an Infant
There are various ways to accomplish a UGI in an infant, and many pediatric radiologists disagree about the details. The following is the way I was taught to perform them. I prefer to have the infant secured to an octagon board (an immobilization device). This allows the radiologist to concentrate on the examination (rather than on keeping the child from wiggling or getting hurt), get images in appropriate positions rapidly, and minimize the radiation dose. Some think that use of the octagon board is inhumane. The babies do dislike being immobilized and often cry; however, in my experience, if time is taken to explain the procedure and its benefits to the accompanying parent, things usually go well. Some radiologists prefer to administer barium orally and some by placing a nasogastric tube. When the child is willing and able to drink, I administer the contrast orally, usually by bottle. In contrast to adults, in whom many images of the stomach and duodenum are obtained to exclude ulcers and cancer, in children, few images are needed. The most important task is to document the position of the DJJ.
I start off with the child feeding in the supine position because they typically are more likely to suck in this position. Once they begin drinking, I obtain an anteroposterior image of the esophagus and then turn them to the lateral position, right side down. I obtain a lateral view of the esophagus and then wait for contrast to pool in the antrum. When the contrast passes through the pylorus and begins to fill the first and second portions of the duodenum, I obtain a lateral view documenting that the pylorus appears normal and that the duodenum courses posteriorly. This is the crucial point in the examination. The infant is then quickly turned supine, and an image is obtained as the contrast courses into the duodenum and proximal jejunum (see Fig. 5-10 ). If the infant is turned supine too early and not enough contrast is in the duodenum, the contrast will not pass leftward over the spine. You can always put the child back into the right-side-down position and get more contrast in the duodenum. If the child is turned supine too late (the worst-case scenario), the contrast will have passed into more distal loops of the jejunum and will obscure visualizing the position of the DJJ. Appropriate timing comes with experience.
The next image that I obtain is an oblique with the left side down, producing an image of the air-filled antrum and bulb. Finally, I obtain an anterior-posterior (AP) image once more contrast has passed into the jejunum to document nondilatation of the jejunum and show that there is no gastroesophageal reflux. Therefore a normal UGI in an infant should consist of only six images. To reduce radiation dose, all of these images can be last-image hold fluoroscopic images rather than true radiographic spot views.
Low Intestinal Obstruction in Neonates
It is not uncommon for neonates to fail to pass meconium because of a distal obstructive process. On radiographs of the abdomen, dilatation of multiple loops of bowel is consistent with a distal obstructive process. The only proximal bowel process that may be associated with multiple dilatated loops of bowel is midgut volvulus, when the bowel dilates secondary to ischemia or infarction. However, these infants are very ill. The neonate who has multiple dilatated loops of bowel on radiographs along with abdominal distention and failure to pass adequate amounts of meconium but is otherwise well on physical exam does not have midgut volvulus as a cause of bowel dilatation and should be evaluated by a contrast enema rather than a UGI. In such a patient without anal atresia on physical examination, the diagnosis is likely to be one of four entities (see Table 5-1 ; Figs. 5-16 through 5-22 ). Of these, Hirschsprung disease and meconium plug syndrome involve the colon, and ileal atresia and meconium ileus involves the ileum.







Neonatal contrast enemas are typically performed using dilute ionic, water-soluble agents and a nonballoon-tip catheter of appropriate size. Barium is not typically used because it can make the evacuation of meconium plugs or meconium ileus more difficult, whereas water-soluble enemas can be therapeutic.
A microcolon is a narrow-caliber colon secondary to disuse; if it is identified on the enema, the cause is likely to be ileal pathology (see Fig. 5-16 ). If contrast is refluxed into a collapsed terminal ileum and the more proximal noncontrast-filled bowel loops are disproportionately dilatated, the diagnosis is likely to be ileal atresia (see Fig. 5-20 ). If the terminal ileum is distended and has multiple filling defects, the diagnosis is meconium ileus (see Fig. 5-21 ).
Meconium Ileus
Meconium ileus occurs secondary to obstruction of the distal ileum due to accumulation of abnormally tenacious meconium. It occurs exclusively in patients with cystic fibrosis and is the presenting finding of cystic fibrosis in approximately 10% of cases. It may be complicated by perforation, volvulus of the bowel, or meconium peritonitis ( Fig. 5-23 ). Radiographs show findings of distal obstruction, which may be associated with bubblelike lucencies secondary to the accumulated meconium or with calcification when perforation is present (see Figs. 5-21 and 5-22 ). Serial water-soluble enemas are commonly used in an attempt to remove the obstruction nonsurgically. There is debate about the optimal contrast agent to use for such serial therapeutic enemas (see Fig. 5-21 ). Table 5-2 shows a summary of the meconium-related gastrointestinal diseases.

Hirschsprung Disease
In contrast enemas performed to examine neonatal distal obstruction, if the proximal colon is distended, the cause of distal obstruction is likely to be colonic secondary to Hirschsprung disease or meconium plug syndrome.
Hirschsprung disease is related to the absence of the ganglion cells that innervate the colon. The denervated colon spasms and causes a functional obstruction. Therefore the affected portions of colon are small in caliber, and the more proximal, normally innervated colon is dilatated secondary to the obstruction. The rectum and a variable amount of more proximal colon are affected in a contiguous fashion; there are no skip lesions. Most patients with Hirschsprung disease (90%) present in the neonatal period with failure to pass meconium (see Fig. 5-17 ). However, patients can present later in life with problems related to constipation. Hirschsprung disease is much more common in boys (4:1) and is associated with Down syndrome in 5% of cases.
When an enema is being performed to evaluate for possible Hirschsprung disease, it is essential to obtain early filling views, collimated to include the rectum and sigmoid colon, in both the lateral and then the frontal position. Findings of Hirschsprung disease include a transition zone from an abnormally small rectum and distal colon to a dilatated proximal colon (see Figs. 5-14 and 5-19 ). In a normal patient the rectum has the largest luminal diameter of the left-sided colon. When the rectum alone is involved by Hirschsprung disease, the sigmoid colon is larger than the rectum (see Fig. 5-17 ). This is referred to as an abnormal rectosigmoid ratio. Another, but less common, finding is fasciculations or saw-toothed irregularity of the denervated segment (see Fig. 5-17 ). If the entire colon is involved by Hirschsprung disease (very rare), the entire colon may appear small in caliber and may mimic a microcolon.
Patients with Hirschsprung disease may present with associated colitis. Therefore, in patients who are suspected to have Hirschsprung disease and are ill, contrast enemas should be avoided.
Definitive diagnosis is obtained by rectal biopsy, and patients are treated by surgical resection of the denervated segment. The transition zone depicted on enema does not always accurately predict where the transition from absent to present ganglion cells occurs histologically.
Meconium Plug Syndrome
Meconium plug syndrome, also referred to as functional immaturity of the colon or small left colon syndrome, is a common cause of distal neonatal obstruction. It is the most commonly encountered diagnosis in neonates who fail to pass meconium. It is thought to be related to functional immaturity of the ganglion cells. As in Hirschsprung disease, the distal colon does not have normal motility, which causes functional obstruction. Unlike Hirschsprung disease, it is a temporary phenomenon and resolves. Although most neonates with meconium plug syndrome are otherwise normal and have no abnormal associations, increased incidence occurs in patients who are infants of diabetic mothers or of mothers who have received magnesium sulfate for eclampsia. In neonates with meconium plug syndrome, there is always concern about underlying Hirschsprung disease, so at many centers, all neonates who have findings of meconium plug syndrome undergo rectal biopsy. In contrast to meconium ileus, there is no significant relationship between meconium plug syndrome and cystic fibrosis.
On contrast enema, multiple filling defects (meconium plugs) are seen within the colon (see Figs. 5-18 and 5-19 ). The right and transverse colons may be more dilated than the left colon (small left colon syndrome; see Figs. 5-18 and 5-19 ), although these findings are variable. Microcolon does not occur. The rectum tends to be normal in luminal diameter, as compared with the rectums in infants with Hirschsprung disease. The enema is often therapeutic; plugs of meconium are commonly passed during or shortly after the enema, and symptoms of obstruction often resolve within hours after the enema.
Esophageal Atresia and Tracheoesophageal Fistula
In esophageal atresia the esophagus is atretic for a variable length, usually at the junction of the proximal and middle thirds of the esophagus. Esophageal atresia can occur in the presence or absence of a tracheoesophageal fistula. The most common type of esophageal atresia is that with a fistulous communication between the distal esophageal segment and trachea. Much less commonly, the fistula can connect the proximal or both the proximal and distal esophageal segments to the trachea. Rarely, tracheoesophageal fistulas can occur in the absence of esophageal atresia; this is called an H-type fistula.
Esophageal atresia presents at birth and is usually encountered by the radiologist after there is failure to pass an orogastric tube. Radiographic findings include a distended, air-filled pharyngeal pouch ( Fig. 5-24 ), with or without an indwelling tube. If there is no abdominal bowel gas, a tracheoesophageal fistula is not present; if there is distal bowel gas, there is a distal fistula. Further imaging, such as with a UGI, is rarely needed. Because the surgery for esophageal atresia is performed through a thoracotomy contralateral to the aortic arch, it is important to determine on which side the arch is located. Often, echocardiography is used.


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree