(T) Primary Tumor | Adapted from 7th edition AJCC Staging Forms. |
TNM | Definitions |
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
T1 | Tumor ≤ 2 cm |
T2 | Tumor > 2 cm but ≤ 5 cm |
T3 | Tumor > 5 cm but ≤ 10 cm |
T4 | Tumor > 10 cm |
(N) Regional Lymph Nodes | |
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph node metastasis |
N1 | Regional lymph node metastasis |
(M) Distant Metastasis | |
M0 | No distant metastasis |
M1 | Distant metastasis |
(G) Histologic Grade | |
GX | Histologic grade cannot be assessed |
G1 | Low grade (mitotic rate ≤ 5 per 50 high-power fields) |
G2 | High grade (mitotic rate > 5 per 50 high-power fields) |
Size of tumor is measured in the greatest dimension. | |
AJCC Stages/Prognostic Groups for Gastric GIST | Adapted from 7th edition AJCC Staging Forms. | |||
Stage | T | N | M | G |
IA | T1 | N0 | M0 | G1 |
T2 | N0 | M0 | G1 | |
IB | T3 | N0 | M0 | G1 |
II | T1 | N0 | M0 | G2 |
T2 | N0 | M0 | G2 | |
T4 | N0 | M0 | G1 | |
IIIA | T3 | N0 | M0 | G2 |
IIIB | T4 | N0 | M0 | G2 |
IV | Any T | N1 | M0 | Any G |
Any T | Any N | M1 | Any G | |
The AJCC stage grouping schema are slightly different for gastric and small bowel GISTs, owing to slightly different biological activities. The staging for gastric GIST should also be used for omentum. | ||||
AJCC Stages/Prognostic Groups for Small Intestinal GIST | Adapted from 7th edition AJCC Staging Forms. | |||
Stage | T | N | M | G |
I | T1 or T2 | N0 | M0 | G1 |
II | T3 | N0 | M0 | G1 |
IIIA | T1 | N0 | M0 | G2 |
T4 | N0 | M0 | G1 | |
IIIB | T2 | N0 | M0 | G2 |
T3 | N0 | M0 | G2 | |
T4 | N0 | M0 | G2 | |
IV | Any T | N1 | M0 | Any G |
Any T | Any N | M1 | Any G | |
The small bowel GIST staging grouping may be applied to esophageal, duodenal, colorectal, and peritoneal GISTs. As there is only minimal data regarding extragastrointestinal GISTs, precise grouping of these rare tumors can be problematic. In cases of extragastrointestinal GISTs and other less common varieties, the staging grouping for small bowel tumors may be used. The T, N, and M criteria reflect imaging findings, whereas tumor grading (G) is based on histologic criteria. | ||||
Gastrointestinal Stromal Tumor Prognostic Grouping | ||
Malignancy Risk | Size Criteria | Histologic Criteria |
Very low risk | < 2 cm | ≤ 5 per 50 HPF |
Low risk | 2-5 cm | ≤ 5 per 50 HPF |
Intermediate risk | < 5 cm | 6-10 per 50 HPF |
5-10 cm | ≤ 5 per 50 HPF | |
High risk | > 5 cm | > 5 per 50 HPF |
> 10 cm | Any mitotic rate | |
Any size | > 10 per 50 HPF | |
From Levy AD et al: Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics. 23(2):283-304, 2003. This system for stratification of aggressive potential in tumors without known metastatic disease was widely used prior to the advent of AJCC TNM staging system and has now been replaced. Size is measured in the greatest dimension. Histologic criteria reflects the number of mitotic figures per 50 high-power fields (HPF). | ||
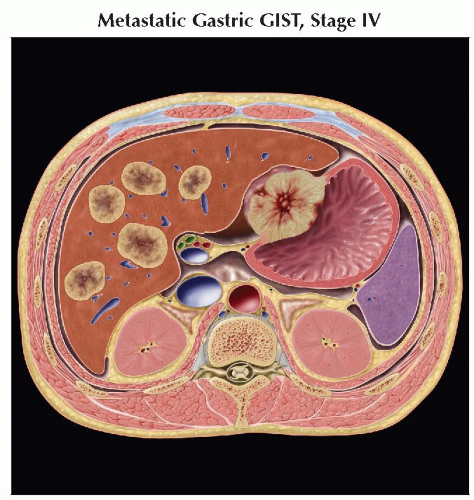
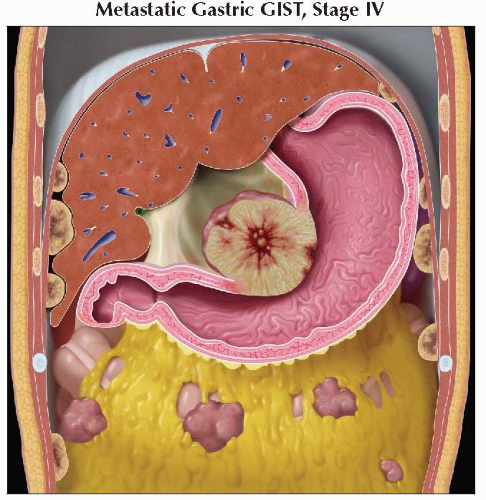
 Coronal graphic shows a small bowel GIST with hepatic and peritoneal metastases. This is a stage IV lesion. |
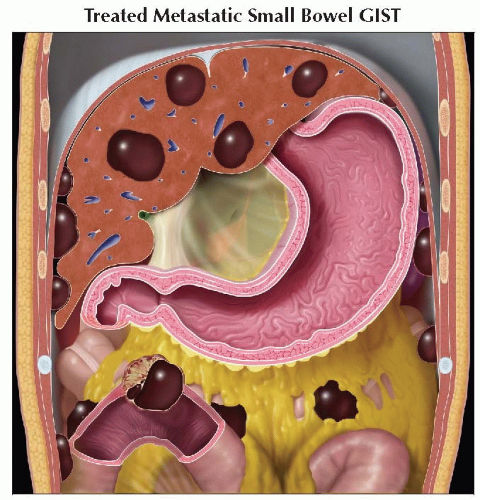 Coronal graphic shows the decrease in size and more cystic nature of primary small bowel GIST after therapy, as well as hepatic and peritoneal metastases. |
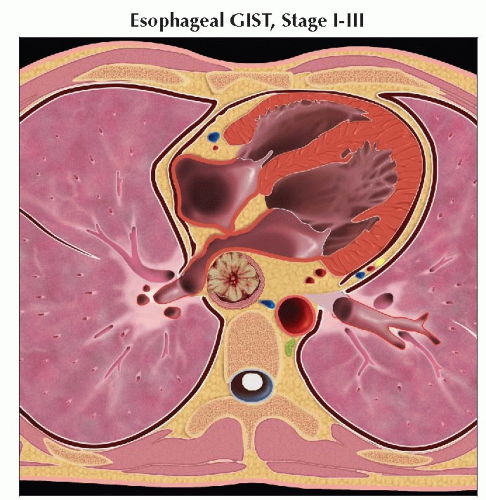 Axial graphic shows mid-esophageal GIST. The small bowel stage grouping should be used in this case. Precise staging is based on lesion size and grade. |
 Axial graphic demonstrates a primary retroperitoneal GIST. The small bowel stage grouping should be used in this case. Precise staging is based on lesion size and grade. |
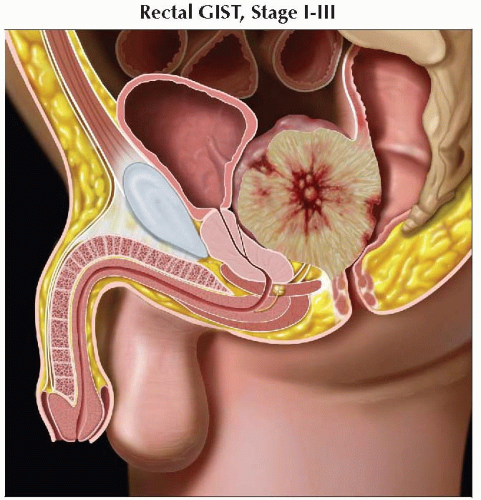 Sagittal graphic shows a rectal GIST. The small bowel stage grouping would be used in this case. Precise staging is based on lesion size and grade. |
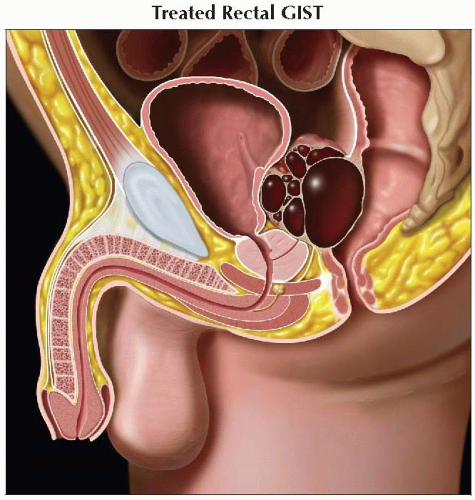 Sagittal graphic shows the decrease in size and more cystic appearance of a rectal GIST after therapy. |
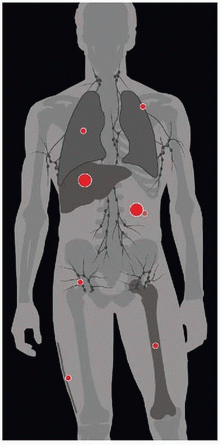
| METASTASES, ORGAN FREQUENCY | |
Liver | 46-65% | |
Peritoneum | 21-41% | |
Retroperitoneum | 4% | |
Lungs | 2-6% | |
Bone | 2-6% | |
Subcutaneous/scar tissue | 2% | |
Pleura | 2% | |
Rare nodal involvement | < 1-6% | |
Most common mesenchymal malignancy of GI tract
Represents about 5-6% of all sarcomas
80% of gastrointestinal sarcomas are GISTs
Accounts for < 1% of all GI malignancies
Historically misdiagnosed smooth muscle tumors
Diagnosed as leiomyomas, leiomyoblastomas, or leiomyosarcomas
Improved immunohistological and electron microscopy techniques allowed for accurate characterization of GISTs after 1983
AJCC TNM staging criteria
Recently established; took effect January 1, 2010
Previously, there was no formal TNM staging system, and risk stratification for metastatic potential was based on tumor size and mitotic rate
Minority (20-30%) of GISTs demonstrate malignant behavior
Smaller, more homogeneous tumors tend to be benign and have lower histologic grade
Tumors < 2 cm rarely demonstrate high histologic grade
Soft tissue sarcoma, distinct from leiomyoma/leiomyosarcoma and nerve sheath tumors
Local spread
Transperitoneal spread
Common pattern of spread, may occur early
Invasion of surrounding structures
Less common than peritoneal seeding
Nodal metastasis
Very rare in GIST
More likely to occur in women and patients < 40 years old
More common in gastric epithelioid/mixed-type ulcerated endoluminal GIST
Distant metastasis
Most commonly metastasizes to liver
Uncommon to spread to lung or other soft tissue sites
Comments
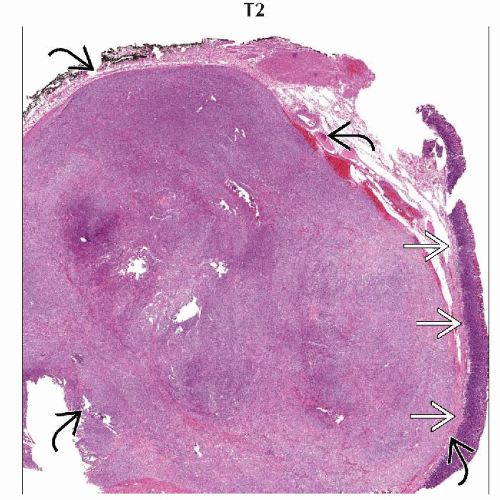
Solid, vascular intramural/submucosal mass
Exophytic growth pattern (not usually infiltrative)
Can demonstrate intra- or extraluminal growth
Tumor may be heterogeneous with variable amount of necrosis/hemorrhage
Can occur anywhere along GI tract
Stomach (50-70%)
Duodenum and small intestine (20-25%)
Colon and rectum (5-10%)
Esophagus (2-5%)
In up to 10% of cases, can occur outside gut (extragastrointestinal GIST)
Retroperitoneum
Mesentery
Omentum
Majority (70-80%) of GISTs are benign
Risk of malignancy can be difficult to estimate
Potential for metastatic spread can be predicted by tumor size and histologic grading
Genetics
c-KIT
Tyrosine kinase oncogene
Encodes for transmembrane growth factor receptor, CD117
More than 90% of patients with GIST have c-KIT mutations
Mutation results in upregulation of tyrosine kinase activity and altered cell growth
PDGFRA
Tyrosine kinase oncogene
Less than 10% of patients with GIST harbor a PDGFRA mutation
Involved with intracellular signaling pathways similar to c-KIT
Etiology
Believed to arise from interstitial cells of Cajal
“Pacemaker” cells of GI tract
Thought to regulate peristalsis
Result of tyrosine kinase oncogene mutation (c-KIT, PDGFRA)
Results in unregulated cellular growth
No described environmental risk factors
Epidemiology & cancer incidence
4,500-6,000 new cases each year in USA
Equal sex predilection
Wide age range at presentation (typically 40-70 years but can occur earlier)
75% of cases in patients > 50 years of age
Median age at presentation: 58-63 years
Associated diseases, abnormalities
Vast majority of GISTs are sporadic and isolated
Carney triad
Association between GIST, pulmonary chondroma, and extraadrenal paraganglioma
Likely sporadic; no known genetic abnormality
Typically epithelioid variant of GIST
Strong female predilection (up to 85%)
Usually occur in stomach and may be multifocal
Minority of patients will have all 3 tumor types at presentation
GIST is often 1st tumor to present
Increased risk of GIST in patients with neurofibromatosis type 1 (NF1)
5-25% of patients with NF1 will develop a GIST
Often multifocal
Predominate in small bowel (as opposed to stomach in sporadic GIST)
< 20% of lesions in NF1 patients with GIST will have typical c-KIT or PDGFRA mutations
Tumor may show S100 positivity (cell marker associated with neural differentiation)
Tend to be of low histologic grade and rarely metastatic
Familial GIST syndromes
Friable, well-circumscribed mass
Unencapsulated but may have pseudocapsule
Larger tumors may demonstrate central necrosis, cystic degeneration, or hemorrhagic components
Measure between 2-30 cm at presentation
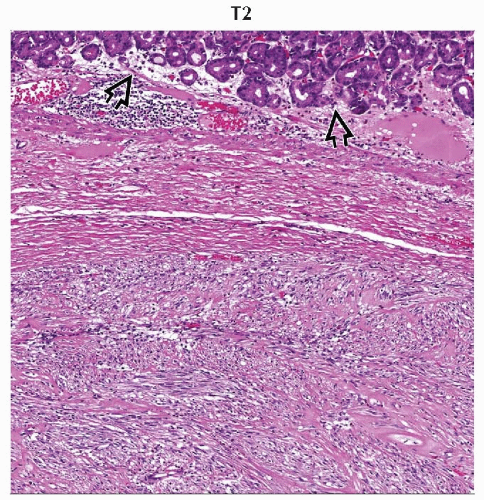
H&E
Spindle cell variant (70%)
Epithelioid variant (20%)
Mixed cell type (10%)
Special stains
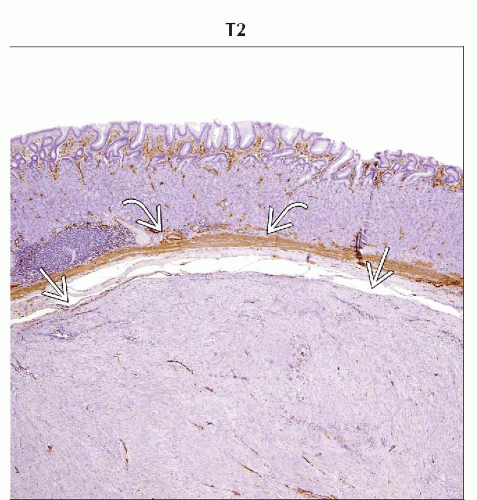
Immunophenotyping essential to differentiate from other mesenchymal tumors
CD117 (c-KIT) positive in nearly 100% of tumors
CD34 positive in 70-80% of tumors
CECT of abdomen and pelvis
Primary imaging modality for tumor detection
Triple-phase examination may be helpful to evaluate tumor vascularity, but single portal venous phase acquisition with oral contrast is usually adequate for diagnosis
Mass arising from gut wall
Intramural growth pattern for small lesions, transmural appearance in larger masses
May also demonstrate intra- or extraluminal predominant growth pattern
Smaller tumors are often well defined
Larger tumors and those of higher grade often have more irregular margins
Invasion of adjacent structures not common and may suggest higher grade lesion
Larger lesion with extraluminal growth may appear as a nonspecific abdominal mass with originating loop of bowel draped along periphery
“Embedded organ” sign
Helpful in identifying organ of origin when large mass is found
Compressed hollow organ adjacent to mass → organ is not site of origin
When part of hollow organ appears embedded within mass → organ is likely site of origin
Avid contrast enhancement
Small tumors show homogeneous enhancement
Central necrosis and cystic changes are common in larger tumors (> 3 cm)
More heterogeneous enhancement may suggest higher grade tumor
Extragastrointestinal GISTs appear as nonspecific soft tissue density enhancing masses
Wide DDx necessitates biopsy
May be difficult to differentiate from metastatic disease; entire gut must be examined closely to exclude a small primary tumor
Upper GI fluoroscopic examination
Smooth submucosal/mural mass lesion
May have irregular contour if necrotic or ulcerated
Necrotic components may communicate with gut lumen
Larger masses may displace adjacent bowel loops
Findings may be suggestive, but cross-sectional imaging necessary for complete characterization and evaluation for metastatic disease
Endoscopy
Often an incidental finding
Mural/submucosal mass
Cross-sectional imaging necessary to evaluate extraluminal extent and presence of metastatic disease
Ultrasound typically not helpful for characterization of primary tumor
Well-marginated masses closely associated with bowel
Often with preservation of typical gut wall signature
Smaller masses are typically homogeneously hypoechoic
May show internal heterogeneity with central anechoic components, representing hemorrhage/necrosis
Association between larger more heterogeneous tumors and higher malignant potential
Hepatic or peritoneal metastatic disease may be identified, though nonspecific in appearance
Hepatic metastatic disease may demonstrate a simple cystic appearance after therapy
MR
Variable appearance based on degree of necrosis/hemorrhage
Solid portions of tumor will typically demonstrate ↓ T1 and ↑ T2 signal
Necrotic/hemorrhagic components will have variable signal intensity
Viable tumor enhances avidly
Hepatic and peritoneal/serosal metastatic disease may be appreciated
Local staging (T)
T staging depends solely on maximum tumor size
T1: Tumor size ≤ 2 cm
T2: Tumor size > 2 cm but ≤ 5 cm
T3: Tumor size > 5 cm but ≤ 10 cm
T4: Tumor size > 10 cm
Diameter may be necessarily estimated in case of ruptured tumor
Depth of gut wall invasion not useful metric as most GISTs are transmural
Invasion of adjacent organs should be described, though does not influence tumor staging
Nodal staging (N)
Lymph node involvement is rare
Nodal dissection usually not indicated at time of surgical resection unless suspicious nodes are seen at imaging
Metastatic staging (M)
Intraabdominal spread (liver or peritoneum/serosa) most common
Adherence of primary mass to liver does not constitute hepatic metastatic disease
Multiple primary GISTs (in setting of familial GIST or NF1) may be difficult to distinguish from metastases
Single omental, peritoneal, or retroperitoneal mass may represent primary GIST vs. metastatic spread from unknown primary
Extragastrointestinal GIST should be diagnosis of exclusion, and great care should be taken to evaluate for gut primary
Liver metastasis
Metastases are frequently hypervascular and may be missed if imaging is performed only during portal venous phase
Histologic grading (G)
Based on number of mitotic figures per 50 highpower fields (HPF)
≤ 5 per 50 HPF = low grade (G1)
> 5 per 50 HPF = high grade (G2)
Imaging modalities
CECT of abdomen and pelvis for staging
Single portal venous phase examination with oral and IV contrast is adequate
Baseline density measurements of mass (in Hounsfield units) should be noted, avoiding overtly necrotic components
Identification of hepatic metastatic disease critical
Careful attention should be paid to mesentery and peritoneum to evaluate for soft tissue nodular implants
While nodal involvement is rare, suspicious lymph nodes should be described
CECT of chest often not necessary as GIST rarely demonstrates extraabdominal spread
PET
Evaluate baseline SUV(max)
Evaluate for additional foci of uptake suggestive of metastatic disease
Contrast-enhanced MR
Most helpful in evaluation of known/suspected rectal GIST
Pelvic sidewall and adjacent organ involvement should be noted
Early peritoneal or hepatic metastatic disease may be detected
No clear consensus on restaging interval, but therapy response can be seen as early as 1 week
CECT is modality of choice for evaluating response to treatment
Size and density (Hounsfield units) of primary tumor should be described and compared with pretherapy values
Size and density (Hounsfield units) of hepatic and mesenteric/peritoneal disease should be reported and compared with pre-therapy values
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree