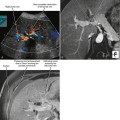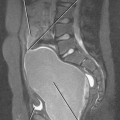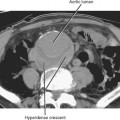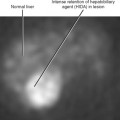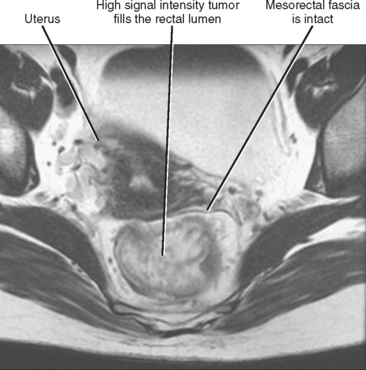
Figure 16-17 Axial T2-weighted magnetic resonance image through the pelvis of a patient with adenocarcinoma of the rectum.
CHAPTER 16 Gastrointestinal Tract
It is unrealistic to think that we can thoroughly address every aspect of gastrointestinal (GI) anatomy and pathophysiology in a textbook of this scope and purpose. Consequently, we focus our discussion on diseases and quandaries likely to be encountered in a typical clinical practice. Our goal is to lead the reader from an imaging finding toward a specific diagnosis or manageable list of likely diagnoses. After a brief discussion of alimentary tract anatomy and imaging modalities, this chapter focuses on diagnostic possibilities for common imaging findings in each section of the alimentary tract, including wall thickening, luminal narrowing, dilatation, ulceration, and masses. This chapter does not always specify a modality when discussing the findings of specific abnormalities. This is to encourage thinking across all modalities, because most radiologists who interpret abdominal studies do not practice in a specific modality, and because many diseases of the alimentary tract manifest on more than one type of imaging study. Furthermore, advances in technology have allowed cross-sectional modalities such as computed tomography (CT) and magnetic resonance imaging (MRI) to create images remarkably similar to fluoroscopic studies (Fig. 16-1).
ANATOMY OF THE GASTROINTESTINAL TRACT
Alimentary Tube
Duodenum
The third portion of the duodenum (the transverse portion) crosses midline from right to left, immediately ventral to the anterior wall of the aorta and dorsal to the origin of the superior mesenteric artery (SMA). This relation explains why aortoenteric fistulas typically involve the third portion of the duodenum. The transverse duodenum is also bordered superiorly by the pancreatic head and, like the second portion of the duodenum, occupies the anterior pararenal space. Contiguity with the pancreas permits frequent involvement of the duodenum with pancreatic cancers and pancreatitis.
Colon and Rectum
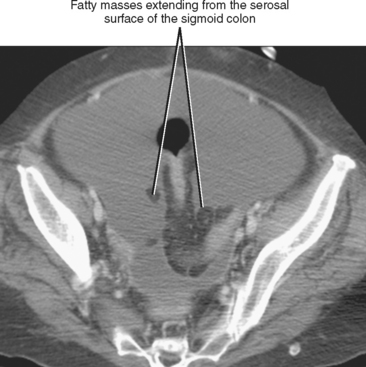
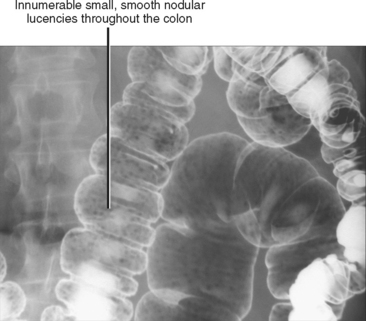
The ascending and descending colon and rectum are extraperitoneal, whereas the transverse and sigmoid colon are intraperitoneal, invested in, and suspended by the transverse and sigmoid mesocolons, respectively. The longitudinal muscle of the colon wall is arranged in three parallel bands (taeniae coli), and the contour of the colon is marked by haustrations. Fatty appendages (appendices epiploicae) attach to the serosal surface of the colon. These are normally inconspicuous unless outlined by ascites (Fig. 16-2). On double-contrast barium examination of the colon, one can sometimes see the fine innominate lines (underdistended colon), transverse folds (contracted colon), and lymph follicles that may have central umbilication (young patients) (Fig. 16-3). Lymph follicles can be associated with lymphoma, carcinoma, and inflammatory processes, and when conspicuous in older patients, should prompt a search for an associated abnormality.
Bowel Wall
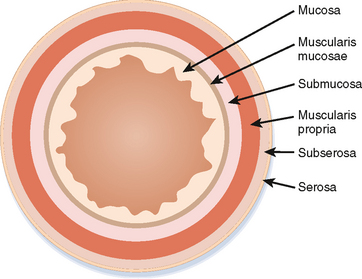
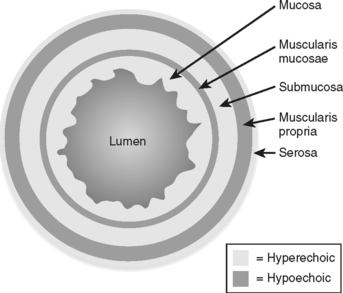
The basic layers of the GI tract wall are illustrated in Figure 16-4. The mucosa is best depicted with double-contrast barium studies, although one can only infer information about the bowel wall with fluoroscopy. Using ultrasound (US) under ideal conditions, one can distinctly identify as many as five bowel wall layers (Fig. 16-5). In many circumstances, however, only a central echogenic area (mucosa and submucosa) surrounded by a hypoechoic rim (muscularis propria) can be distinguished.
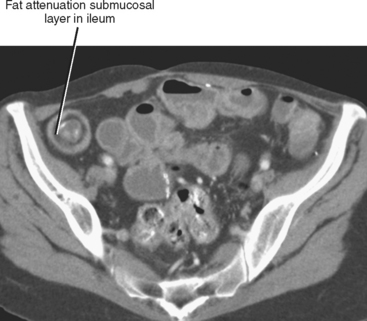
The normal gastric wall is approximately 5 mm thick as measured between rugal folds in a distended state. The valvulae conniventes of the small bowel are normally less than 2 to 3 mm thick in distended loops, and the normal small bowel wall is only 1 to 2 mm thick when the lumen is sufficiently distended (diameter ≥ 2 cm). The normal colon wall thickness also measures only a few millimeters when adequately distended. Once considered an indicator of inflammatory bowel disease (Fig. 16-6), intramural fat can occur normally in the small intestine and colon of obese patients. This fatty layer becomes less conspicuous with luminal distention.
Bowel Rotation
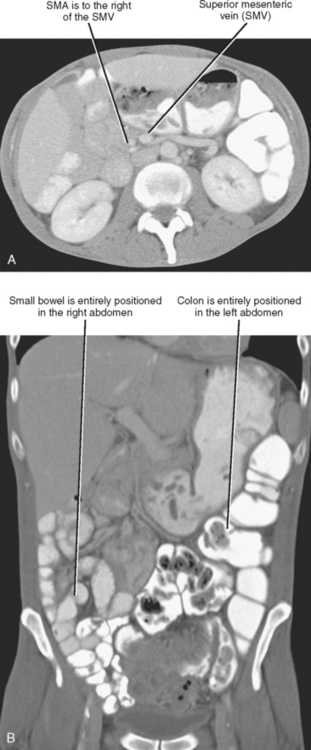
Normal orientation of the intestinal tract occurs only after the embryologic gut completes a complex series of maneuvers. Failure of any one of these steps results in a variety of congenital abnormalities of the bowel. These developmental maneuvers include elongation of the gut and suspending mesentery, herniation of the gut into the base of the umbilical cord, rotation, and return of the developing gut to the abdominal cavity. It is important to recognize when the process of gut rotation and fixation has gone awry. Box 16-1 lists imaging findings that suggest a gut rotational abnormality. The clinical significance of abnormal rotation and fixation of the bowel is the increased risk for intestinal volvulus, especially in neonates and infants.
IMAGING MODALITIES
Radiography (Plain Films)
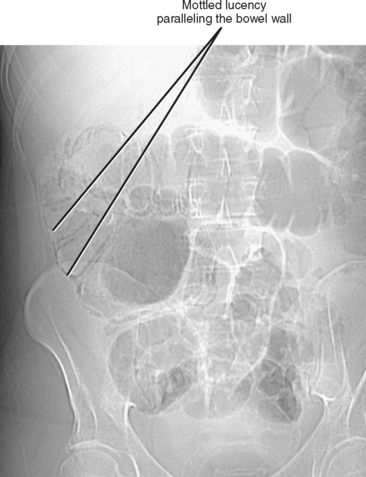
Radiographs are still widely performed for the evaluation of abdominal complaints. Supine portable radiographs can confirm enteric tube placement, identify calcifications and foreign bodies, suggest a mass or organomegaly, and evaluate for obstruction. The combination of supine and upright radiographs is generally preferred to supine images alone for detection of extraluminal (i.e., intraperitoneal, intramural, or intravascular) gas and for assessment of the bowel gas pattern. Wall thickening can be inferred on abdominal radiography, although the bowel wall is not directly visible in the absence of peritoneal gas (Fig. 16-8). Bowel obstruction causes dilated gas-filled bowel loops proximal to the obstruction, with a lack of gas or bowel contents distal to the obstruction. Dilated fluid-filled bowel loops can escape radiographic detection, although obstruction can be suspected when bubbles of gas collect between mucosal folds (“string-of-pearls sign”).
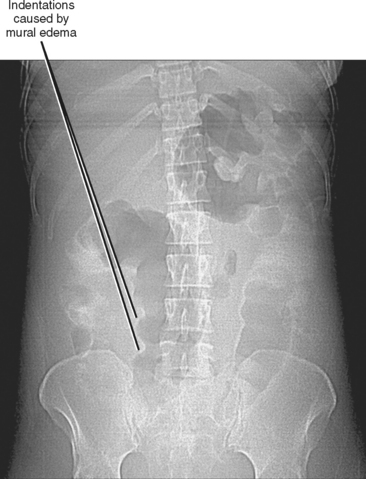
Figure 16-8 Supine abdominal radiograph of a patient with ulcerative colitis demonstrating thumbprinting of the colon.
Pneumatosis intestinalis can be a subtle finding on radiographs, imparting a bubbly appearance to the bowel that is often mistaken for stool (Fig. 16-9). The presence of pneumatosis should prompt a search for portal venous gas, best seen overlying the periphery of the liver, often on the left of a supine patient. Often, radiographs will be inconclusive in the setting of abdominal pain, and further imaging evaluation will be necessary. Because abdomen radiographs can be the first imaging study for a patient with abdominal pain, timely and accurate interpretation can be critical to patient management and outcome.
Fluoroscopy
Clinical Utility
In reality, fluoroscopy plays a limited, but nonetheless important, role in GI problem solving (Box 16-2). As with other imaging modalities, such as US, the problem-solving yield of fluoroscopy can be improved by taking an interactive approach. The timely acquisition of spot films, attention to motility, appropriate patient positioning, and application of compression can markedly improve the diagnostic yield of fluoroscopic examinations. The appropriate usage of specialized techniques such as peroral pneumocolon and enteroclysis can further increase the diagnostic yield.
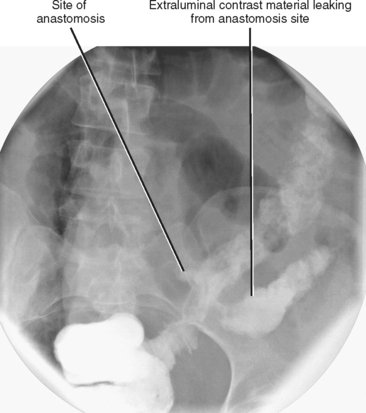
There are some scenarios in which fluoroscopy can be potentially harmful. These include toxic megacolon and typhlitis. Water-soluble contrast is preferable to barium in cases of suspected bowel perforation, obstipation, chronic intestinal pseudoobstruction, fistula evaluation, and before abdominal surgery or endoscopy.
Fluoroscopic Findings
Box 16-3 and Table 16-1 list key findings visible with GI fluoroscopy. Of course, one must also remember to inspect regions and findings beyond the confines of the GI tract, such as abnormal gas collections, calcifications and other abnormal opacities, organ outlines, and skeletal abnormalities.
Table 16-1 Fluoroscopic Findings and Their Significance
| Finding | Possible Significance |
|---|---|
| Thickened folds (single or double contrast) | Inflammation, infection, neoplastic infiltration, intramural hemorrhage, or submucosal edema |
| Radiating folds (single or double contrast) | Normal gastric cardia or pylorus, gastric or duodenal ulcer or ulcer scar |
| Linear density (double contrast) | Linear ulcer, edge of a protruding lesion, edge of an extrinsic lesion, extrinsic structure viewed through bowel, or coaptation artifact |
| Ring shadow (double contrast) | Gas-filled diverticulum, polyp surrounded by gas, gas-filled ulcer, gas bubble, or extrinsic structure viewed through bowel (e.g., pedicle) |
| Double ring shadow (double contrast) (Fig. 16-11) | Polyp on a stalk viewed end-on creates a double ring appearance; this has been referred to as the “Mexican hat sign”; do not confuse this with a barium droplet hanging from sessile polyp or barium pooled in an ulcer crater or diverticulum; in these latter cases, the center of the inner ring will be uniformly dense rather than lucent |
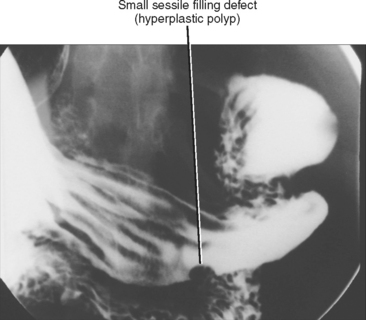
| Bowler hat (double contrast) (Fig. 16-12) | Sessile polyp (dome of hat points to lumen) or diverticulum (dome of hat points away from lumen) |
| Focal round density (double contrast) | Stalactite phenomenon (barium dripping from a protruding lesion of the nondependent surface), ulcer, diverticulum, barium precipitate, barium trapped in mucosal fold or pit, or extrinsic density (e.g., calcification) |
| Filling defect (single contrast) (Fig. 16-13) | Mucosal or submucosal mass, food, stool, foreign body, bubble, blood clot, redundant or nodular mucosa |
| Bull’s-eye/target lesion (single or double contrast) | Hematogenous metastasis, ulcerated submucosal tumor, aphthous lesion, or ectopic pancreatic rest |
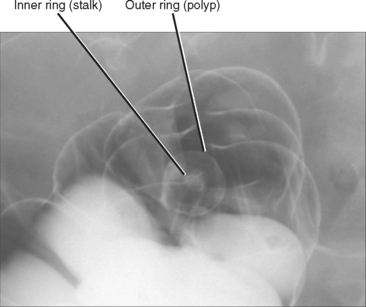
Figure 16-11 Image from a double-contrast barium enema demonstrates a pedunculated polyp creating a “double ring shadow.”
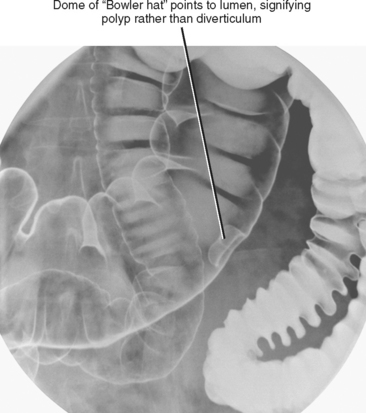
Figure 16-12 Image from a double-contrast barium enema demonstrates a sessile polyp of the transverse colon.
Ultrasound
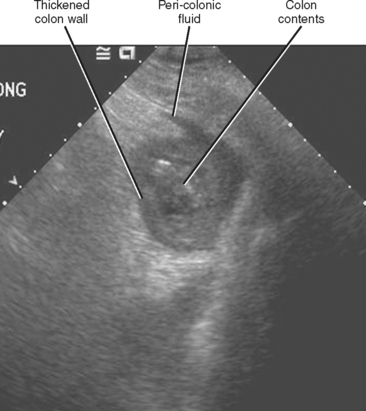
In the United States, ultrasound is rarely used as a stand-alone technique for assessment of bowel. In most general radiology practices, it is more likely that a bowel abnormality will be discovered incidentally during an abdominal sonographic survey than as the result of a directed search (Fig. 16-14). It is perhaps most important that one be able to distinguish between normal and abnormal bowel with US, because additional diagnostic tests will likely be necessary before a definitive diagnosis can be established.
When bowel wall thickening is present, one should attempt to distinguish between short-segment thickening (more worrisome for a malignant process) and longsegment thickening (more likely with benign processes such as inflammation or infection). Bowel wall tumors are usually hypoechoic. Increased echogenicity in the fat adjacent to bowel may be a sign of inflammation. Gas trapped in ulcerated mucosa produces linear echogenic foci often associated with ring-down artifact. Pneumatosis produces echogenic foci in the bowel wall that can shadow. The normal bowel wall shows minimal signal with color Doppler. Completely absent flow with color Doppler can be a sign of intestinal ischemia, whereas increased flow suggests inflammation or neoplasia.
Computed Tomography
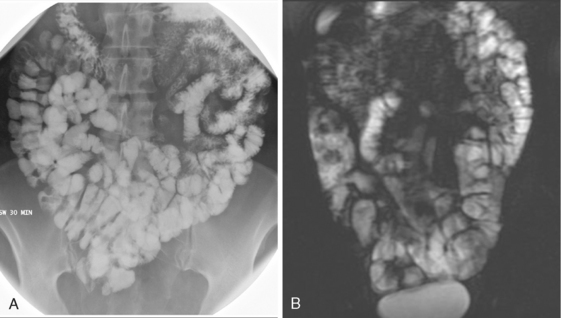
With appropriate patient preparation, protocol design, and reconstruction techniques, CT is an excellent means of assessing the GI tract (Box 16-4). CT excels at the detection of inflammatory and neoplastic processes that affect the bowel, even when patient preparation and cooperation are less than optimal. CT has replaced other methods of detecting the presence, site, and cause of bowel obstruction. CT enterography (without small-bowel intubation) and enteroclysis (with small-bowel intubation) are used increasingly to evaluate small-bowel disorders, and CT colonography may soon become the preferred method of screening patients for colon polyps and cancer (Fig. 16-15). Currently, most nonpolypoid mucosal processes, small vascular malformations, motility disorders, and malabsorptive states cannot be reliably and routinely diagnosed with CT.
Magnetic Resonance Imaging
Indications for Magnetic Resonance Imaging of the Bowel
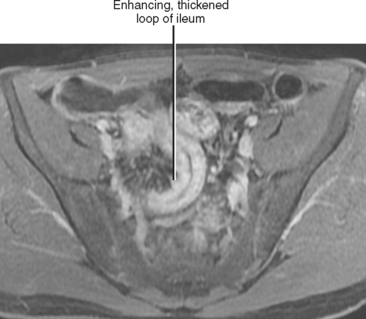
MRI of the bowel has a few significant advantages over CT that may increase utilization for evaluation of the GI tract. These include improved contrast conspicuity and lack of ionizing radiation. However, MRI examinations take significantly longer than the typical CT scan to perform and require greater patient cooperation. As scan times continue to shorten and expertise continues to grow, MRI will doubtless play a larger role in evaluating the GI tract in the near future.
Sequences useful for imaging bowel include single-shot echo train spin-echo sequences, balanced steady-state free precession sequences, and two- or threedimensional gadolinium-enhanced fat-suppressed gradient-echo sequences. Administration of an antiperistaltic agent immediately before contrast-enhanced imaging improves image quality. When performing gadolinium-enhanced imaging to evaluate the bowel wall, a negative contrast agent (dark on T1) that distends the bowel lumen is helpful. A variety of intraluminal contrast agents have been recommended for this purpose, including water, dilute barium, and polyethylene glycol. Additives such as mannitol, Psyllium husk, and locust bean gum have been used to improve bowel distention. Although expertly performed MRI of a cooperative patient is equivalent to CT for evaluation of many suspected GI abnormalities, there are a few indications for which MRI may be preferable to CT (Box 16-5).
Nuclear Medicine
Nuclear scintigraphy has a limited but important role in evaluating the GI tract. Table 16-2 lists potential applications to consider.
Table 16-2 Potential Indications for Nuclear Scintigraphy of the Gastrointestinal Tract
| Radiopharmaceutical | Application |
|---|---|
| Tc-99m sulfur colloid | |
| Tc-99m red blood cells | |
| Tc-99m pertechnetate | |
| Tc-99m CEA-scan | |
| In-111 pentreotide |
Tc-99m, Technetium 99m.
Positron emission tomography (PET) utilizing F-18 fluorodeoxyglucose (18F-FDG) is revolutionizing oncologic imaging. Because cancer cells often demonstrate higher metabolic activity than normal cells, the uptake of glucose by many cancer cell types is generally greater. 18F-FDG is a glucose analog that is handled in much the same way by cells as glucose. Within the GI tract, PET imaging with 18F-FDG is used primarily to stage and assess response to therapy for esophageal and colorectal carcinoma. PET may also detect malignant tumors from non-GI sites that secondarily involve the bowel.
ABNORMALITIES OF THE STOMACH
Thickening
Normally the gastric wall is thinner in nondependent segments of the stomach that are well distended by gas. In addition, adherent debris that may add to the appearance of gastric wall thickening typically remains within the fluid-filled dependent portions. When wall thickening involves not only dependent, fluid-filled parts of the stomach but also nondependent, gas-filled portions on a CT or MRI scan, an underlying abnormality should be suspected (Fig. 16-18).
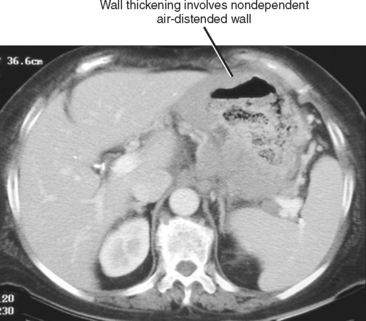
Figure 16-18 Enhanced axial computed tomographic scan through the abdomen of a patient with B-cell lymphoma involving the stomach.
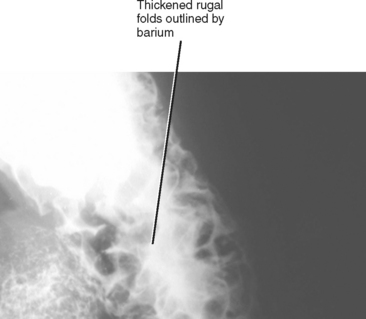
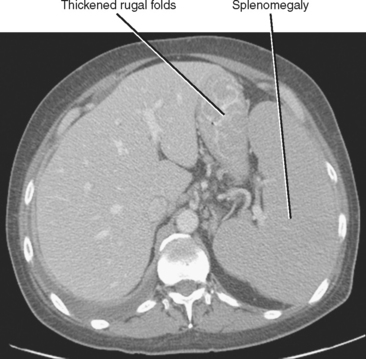
Thickening of the rugal folds has an extensive differential diagnosis (Table 16-3). Fortunately, many of the entities that cause rugal fold thickening are extremely rare, and many of the common causes will have other imaging findings that allow a specific diagnosis to be made.
Table 16-3 Causes of Rugal Fold Thickening
| Causes | Helpful Distinguishers |
|---|---|
| Common Causes | |
| Underdistention | |
| Gastritis (most often Helicobacter pylori) | |
| Pancreatitis | |
| Portal hypertension | |
| Varices | |
| Lymphoma (Figs. 16-19 and 16-20) | |
| Rare Causes | |
| Zollinger–Ellison syndrome | |
| Ménétrier disease | |
| Eosinophilic gastritis | |
| Amyloidosis | |
| Sarcoidosis | |
CT, Computed tomography; MR, magnetic resonance; UGI, upper gastrointestinal.
Gastric adenocarcinoma can cause focal or diffuse gastric wall thickening, although the normal rugal fold pattern is typically not maintained at the site of tumor. Scirrhous carcinoma infiltrates the submucosa of the stomach, resulting in some combination of wall thickening, luminal narrowing, and reduced distensibility. Gastric lymphoma can have a variety of appearances including diffuse gastric wall thickening (see Fig. 16-18).
Luminal Narrowing
Gastric narrowing is often more apparent on barium examination of the stomach than on cross-sectional imaging studies, because most causes of gastric narrowing also result in reduced distensibility and hypomotility. The antrum is the most commonly narrowed gastric segment, and wall thickening often accompanies luminal narrowing. Clinical history plays an important role in distinguishing causes of gastric narrowing (e.g., history of caustic ingestion, radiation treatment, known primary neoplasm). Table 16-4 lists some causes of gastric narrowing.
Table 16-4 Causes of Gastric Narrowing
| Category | Disease Process |
|---|---|
| Neoplastic | |
| Inflammatory | |
| Infectious | |
| Extrinsic compression | |
| Other |
Ulceration
Most gastric ulcers are found along the posterior wall of the antrum or body, or along the lesser curvature of the stomach. Anterior wall or greater curvature ulcers account for about 15% of gastric ulcers. Of course, this statistic is not particularly useful to the radiologist faced with one particular ulcer on a fluoroscopic study. It is far more useful to know whether to recommend endoscopy for further evaluation when encountering a gastric ulcer. In general, endoscopy should be pursued if any ulcer is less than unequivocally benign in appearance (Table 16-5). Although the other signs are useful from a statistical standpoint, only the rarely seen Hampton line is believed to be an unequivocal sign of benignity.
Table 16-5 Features Suggestive of Benign versus Malignant Gastric Ulcers
| Feature | Benign | Malignant |
|---|---|---|
| Location | Lesser curvature and distal stomach more common | Greater curvature and proximal stomach more common |
| Margins | Sharp, round | Irregular, elevated |
| Folds | Smooth, extend to edge of crater | Nodular, amputated, do not extend to crater edge |
| Mucosa | Area gastricae intact | Area gastricae distorted or obliterated |
| Profile | Projects beyond expected lumen contour | Projects within expected lumen contour |
| Signs | Hampton line* | Carman meniscus sign† |
| Clinical course | Heals with appropriate peptic ulcer therapy | Persists or progresses despite appropriate therapy for peptic ulcer disease |
* Hampton line refers to a thin, straight lucent line across the neck of a contrastfilled ulcer representing intact mucosa.
† Carman meniscus sign is created when the heaped-up edges of a large shallow malignant ulcer are apposed during compression, trapping barium in a collection that is convex to the gastric lumen.
Erosions are small, superficial linear or round ulcerations that often occur on the crests of gastric folds. Erosions are typically multiple and often demonstrate a radiolucent halo of edematous mucosa on double-contrast barium examinations. Box 16-6 lists some causes of gastric erosions. Note that many of the entities that cause erosions also can result in gastric wall thickening and narrowing when advanced.
Masses
Pseudotumors
The stomach is a common site for a false-positive interpretation regarding a mass. Some common locations for gastric pseudotumors include the region of the gastric cardia, along the proximal lesser curvature, and in the distal antrum and pyloric region. Most often, the appearance of a mass is related to redundant, contracted, or nondistended gastric wall, and the issue can be satisfactorily resolved with further distention of the stomach. Ingested material or bezoar may also give the false impression of a gastric mass (Fig. 16-21). A pseudotumor can change dramatically in position, often does not have a consistent connection with the gastric wall, and may fail to demonstrate enhancement after intravenous contrast administration on CT and MR images. A true gastric neoplasm is a persistent finding on multiple phases, acquisitions, or examinations that has a consistent communication with the gastric wall and demonstrates enhancement. Movement alone is not a helpful sign because pedunculated gastric masses can change position. Unlike some bezoars, gastric neoplasms do not contain gas. A pancreatic rest can simulate an ulcerated submucosal mass. Ectopic pancreatic tissue usually occurs along the greater curvature of the distal gastric antrum or in the proximal duodenum. Pancreatic rests are usually small (1 cm or less), broad based, smooth, and have a central umbilication related to the primitive ductal system as seen on barium studies.
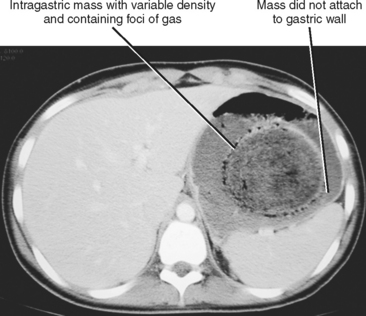
Figure 16-21 Axial image from a contrast-enhanced computed tomographic scan through the stomach of a patient with a large bezoar.
Gastric Polyps
Gastric polyps are common mucosal masses of the stomach. Gastric polyps can be adenomatous, hyperplastic, or hamartomatous (Fig. 16-22). Most gastric polyps are hyperplastic, whereas in the duodenum, most polyps are adenomatous. Hyperplastic polyps are often small (<1 cm), smooth, sessile, and multiple. Adenomatous polyps are frequently solitary and more likely to be large (>2 cm) and pedunculated (Table 16-6).
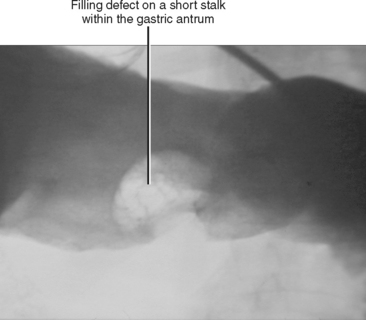
Figure 16-22 Spot film from a single-contrast upper gastrointestinal examination demonstrates an antral polyp.
Table 16-6 Adenomatous versus Hyperplastic Gastric Polyps
| Feature | Adenomatous | Hyperplastic |
|---|---|---|
| Size | >1 cm | <1 cm |
| Shape | Lobulated | Smooth |
| Number | Solitary | Multiple |
| Base | Sessile or pedunculated | Sessile |
Submucosal Mass
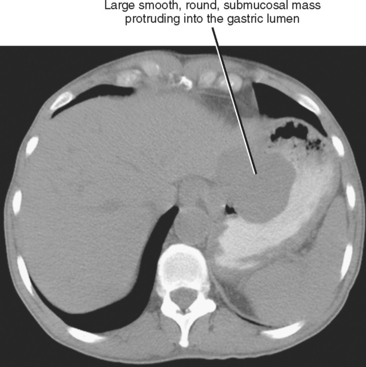
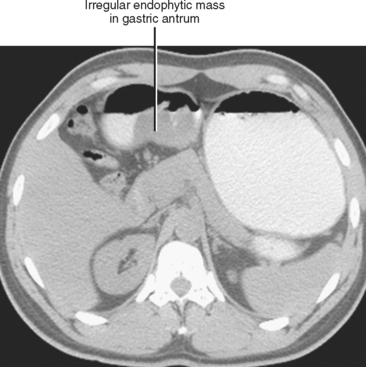
Submucosal masses typically have borders that are mildly obtuse with respect to the gastric wall, although some submucosal masses will appear pedunculated, potentially creating confusion with gastric polyps. Most benign submucosal masses are smooth with intact overlying gastric mucosa, although central ulceration or umbilication can occasionally create a bull’s-eye appearance. Definitively characterizing a submucosal mass with imaging is difficult in most cases. Occasionally, some clues as to causative factors exist. Lipomas and lymphangiomas are compressible and can be seen to change shape. Lipomas are fat density, whereas lymphangiomas are cystic on CT images. A lesion that contains phleboliths represents a hemangioma. A solitary, smooth, round, submucosal soft-tissue mass or a large, exophytic, necrotic mass are statistically most likely to represent leiomyoma or gastrointestinal stromal tumor (GIST) (Fig. 16-23). Box 16-7 lists some causes of submucosal mass.
Common Malignant Gastric Tumors
GASTROINTESTINAL STROMAL TUMOR
GISTs are histologically distinct from leiomyomas, although this distinction cannot be made with imaging. Likewise, the distinction between benign or malignant is difficult unless metastatic disease or frank invasion is present. Most GISTs are sporadic, but these tumors can be associated with neurofibromatosis (NF-1) and Carney triad (gastric GIST, pulmonary chondroma, extraadrenal paraganglioma). GISTs are usually solitary, often large, round tumors of the gastric wall. They can appear primarily exophytic (common), intramural, or endophytic (i.e., bulging into gastric lumen [least common]). GISTs are often necrotic, demonstrating mucosal ulceration or deep cavitation that can communicate with the gastric lumen. Up to 25% of lesions demonstrate calcifications on CT. GISTs usually are of intermediate signal intensity on T1-weighted MRI and intermediate to low signal intensity on T2-weighted MRI. Areas of central necrosis demonstrate increased signal intensity on T2-weighted images. Enhancement after intravenous contrast administration is variable on CT and MRI (necrotic areas do not enhance). GISTs tend to be 18F-FDG avid. Therefore, PET is a useful modality for documenting early response to imatinib. Malignant tumors can spread to liver, peritoneum, and rarely, lymph nodes. Metastatic foci often resemble the primary tumor, although metastases treated with imatinib can mimic cysts.
ADENOCARCINOMA
Most gastric cancers are adenocarcinomas. Adenocarcinomas can be polypoid, plaquelike, endophytic, exophytic, or infiltrative. Unlike stromal tumors, most adenocarcinomas present as endophytic masses or irregular focal wall thickening (Fig. 16-24). Ulceration is relatively common. Scirrhous carcinoma results in a thickened nondistensible stomach (linitis plastica appearance). Gastric cancer can be associated with malignant ascites, peritoneal nodules, or regional lymphadenopathy.
LYMPHOMA
Lymphoma often presents with thicker and more diffuse circumferential involvement of the stomach than other tumors (see Fig. 16-18). Despite this, the stomach involved with lymphoma often remains distensible. As elsewhere in the body, lymphoma is a great mimicker of other diseases. When presenting as a large, solitary, ulcerated mass, lymphoma can be indistinguishable from a GIST. Hodgkin’s lymphoma can be scirrhous or present as thickened rugal folds. MALT lymphoma is a low-grade B-cell lymphoma associated with Helicobacter pylori infection. MALT lymphoma can present as multiple round, confluent nodules. Lymphadenopathy in a distribution other than the gastric drainage is a clue that a gastric mass represents lymphoma, although lymphadenopathy is not a conspicuous feature of MALT lymphoma. Gastric outlet obstruction is uncommon with lymphoma.
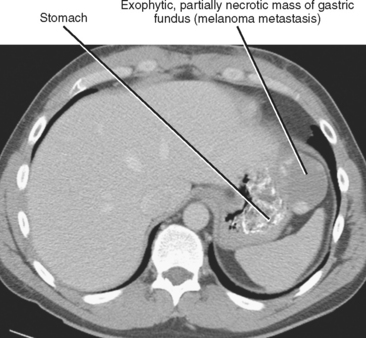
SECONDARY TUMORS
Malignant tumors can spread to the stomach via hematogenous spread, direct spread from contiguous organs, and peritoneal spread (Fig. 16-25). Breast cancer, lung cancer, and melanoma are among the more common malignant neoplasms to metastasize to the stomach. Hematogenous metastases can mimic primary gastric tumors such as adenocarcinoma or GIST, although the classic appearance is one of multiple centrally ulcerated submucosal masses (bull’s-eye lesions). Breast cancer involving the stomach can cause a linitis plastica appearance. Pancreatic cancer and colon cancer can spread to the stomach directly, whereas other tumors can involve the stomach via the gastric mesenteries. Direct invasion of the stomach from a nongastric primary results in spiculation and/or tethering of the involved wall with distortion of the mucosal folds. CT reveals a soft-tissue mass obliterating the fat planes between the stomach and the organ of origin.