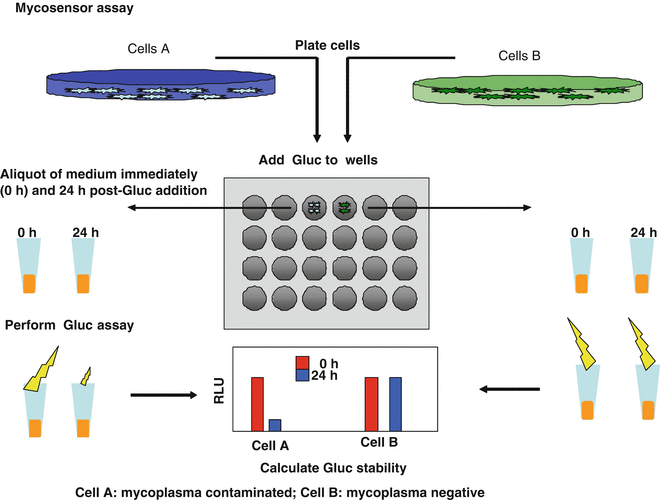
(1)
Experimental Therapeutics and Molecular Imaging Laboratory, Department of Neurology, Neuroscience Center, Massachusetts General Hospital, Boston, MA, USA
(2)
Program in Neuroscience, Harvard Medical School, Boston, MA, USA
(3)
Department of Neurosurgery, Leiden University Medical Center, Leiden, The Netherlands
(4)
Notre Dame University, Barsa, Lebanon
Abstract
Mycoplasma contamination in mammalian cell culture is a common problem with serious consequences on experimental data, and yet many laboratories fail to perform regular testing. In this chapter, we describe a simple and sensitive mycoplasma detection assay based on the bioluminescent properties of the Gaussia luciferase reporter.
Key words
Mycoplasma contaminationMycoplasma detection assayGluc mycosensorCell culture contaminationAntibioticsBioluminescence reactionAuthors Hannah Degeling and Sarah S. Bovenberg have contributed equally to this chapter.
1 Introduction
Mycoplasmas are common contaminants of mammalian cell culture; due to their small size and the absence of a rigid cell wall, they have the ability to pass through most bacterial filters. In contrast to other contaminants such as fungi and bacteria, mycoplasma contamination is impossible to detect by eye, since the contamination does not influence the color of the cell culture medium, the pH, the turbidity, nor the odor. Mycoplasmas are also not visible under the regular light microscopes in cell culture laboratories. Furthermore, the fact that mycoplasma can grow both intracellular and extracellular greatly contributes to their resistance to several antibiotics in the extracellular cell culture medium, such as penicillin and streptomycin [1]. Due to these issues, mycoplasma contamination rates in mammalian cell culture laboratories have been found to be as high as 70 % [1–3].
Mycoplasma contamination can have a tremendous effect on host cells. For instance, mycoplasma can interfere with numerous cellular processes including cell metabolism, proliferation, gene expression, and function [2–7]. Therefore, all cell culture laboratories should test cell cultures for mycoplasma contamination on a regular basis. The ideal detection method for mycoplasma contamination should be simple to perform, sensitive, specific, rapid, inexpensive, and suitable for testing of numerous cell cultures simultaneously.
In this chapter, we describe a simple mycoplasma detection assay based on degradation of the Gaussia luciferase (Gluc) reporter protein. Typically, in conditioned medium of cells under regular culture conditions, Gluc has a half-life of >7 days [8]. The half-life of Gluc is tremendously decreased in the presence of mycoplasma contamination, and therefore the level of decline in Gluc activity correlates to mycoplasma infection rate [9]. This mycoplasma-specific decrease has been confirmed by several assays including Western blot analysis, which showed Gluc protein degradation over short period of time (2–24 h) only in the presence of mycoplasma contamination. We reasoned that this phenomenon could be used as a sensitive and specific biosensor to monitor the mycoplasma contamination in mammalian cells (Mycosensor) [9].
Around 90–95 % of all mycoplasma contamination in mammalian cell cultures is caused by either M. orale, M. hyorhinis, M. arginini, M. fermentans, M. hominis, or A. laidlawii [1]. The Gluc mycosensor has been confirmed on three of these commonly isolated mycoplasma strains including Mycoplasma fermentens, Mycoplasma hominis, and Mycoplasma orale. Importantly, this Mycosensor showed to be more sensitive in detecting mycoplasma contamination as compared to a commercially available bioluminescent-based assay and is amenable to high-throughput applications [9]. Alternative Mycoplasma detection methods are available including mycoplasma DNA amplification by polymerase chain reaction, which is sensitive but prone to errors; by traditional bacterial cell culture, which is very time consuming [10]; or by commercially available kits which have low sensitivity and are costly. The Gluc Mycosensor, however, is easy to use, sensitive, and especially suited for testing of numerous cell quantities simultaneously. Nevertheless, we recommend in all cases to combine two different detection methods to achieve and maintain total mycoplasma clearance in the cell culture hoods.
2 Materials
2.1 Gluc Recombinant Protein
1.
Gluc recombinant protein (Nanolight).
Or, for the preparation of Gluc recombinant protein:
2.
cDNA encoding Gluc (Nanolight), and N-terminal pelB periplasmic signal sequence, and a C-terminal 6-His tag in pET26b(+) vector (Novagen).
3.
HMS174 competent bacterial cells and LB containing 30 μg/mL kanamycin.
4.
IPTG Bugbuster Master Mix containing Benzonase (Novagen).
5.
NJ45 μm syringe filter nickel charged resin column (Novagen).
6.
Binding buffer: 0.5 M NaCl, 20 mM Tris–HCl, 5 mM imidazole, pH 7.9.
7.
Wash buffer: 0.5 M NaCl, 20 mM Tris–HCl, 60 mM imidazole, pH 7.9.
8.
Elution buffer: 0.5 M NaCl, 20 mM Tris–HCl, 1 M imidazole, pH 7.9.
9.
Coelenterazine and a luminometer.
10.
3,500 MW cut-off dialysis tubing (Fisher Scientific).
11.
Bradford assay.
12.
SDS-PAGE, NuPAGE„¥ 10 % Bis–Tris gel and coomassie blue staining (Invitrogen).
2.2 Gluc-Containing Medium
1.
293 T human fibroblast cells.
2.
Lentivirus vector expressing Gluc and the enhanced green fluorescent protein (GFP) under the control of CMV promoter.
3.
Polybrene.
2.3 Cell Culture
3 Methods
Be aware that all materials used for cell culture and the Mycosensor assay should be stored and used under sterile conditions to avoid contamination during the Gluc incubation period. An overview of the complete Gluc Mycosensor assay protocol is presented in Fig. 1.