Agent
Organ
Toxicity
Bevacizumab
GI
GI perforation, pneumatosis, bleeding
Bleomycin
Pulmonary
Interstitial infiltrates/fibrosis
Cisplatin
Vascular
Arterial and venous thrombosis
Etoposide
Secondary malignancy
Leukemia
Gemcitabine
Pulmonary
Capillary leak syndrome, diffuse alveolar damage, alveolar hemorrhage
Vascular
Arterial and venous thrombosis
Interleukin–2
Pulmonary
Interstitial edema, capillary leak syndrome
Methotrexate
Pulmonary
Hypersensitivity pneumonitis, COP, noncardiogenic pulmonary edema
Sunitinib/sorafenib
GI
Enteritis
Temsirolimus/everolimus
Pulmonary
Ground glass infiltrates, COP
Renal Cell Cancer
Treatment Options for Metastatic Renal Cell Cancer
The treatment of advanced renal cell carcinoma (RCC) has undergone major advances in recent years as a result of the development of new targeted molecular agents [2–5]. These include the vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitors (TKI), the mTOR inhibitors, and the VEGFR monoclonal antibodies. The three orally administered VEGFR TKI agents that are U.S. Food and Drug Administration approved for the treatment of advanced RCC are sunitinib, sorafenib, and pazopanib. These agents inhibit multiple tyrosine kinases involved in cell proliferation, including VEGFR and platelet-derived growth factor receptors (PDGFR), which are activated in the majority of sporadic clear cell RCCs [5, 6]. The mTOR inhibitors, temsirolimus and everolimus, inhibit a key intracellular pathway represented by a protein kinase known as the mammalian target of rapamycin (mTOR) [6]. Inhibition of this key intracellular pathway results in interruption of cell proliferation, cell growth, and tumor angiogenesis. The anti-VEGF monoclonal antibody bevacizumab exerts its anti-angiogenic effects by binding to the circulating VEGF. Current evidence supports the use of sunitinib, pazopanib, and the combination of bevacizumab plus α-interferon for treatment-naïve patients with good- and intermediate-risk advanced RCC and temsirolimus for treatment-naïve patients with poor-risk RCC. Standard second-line therapies include sorafenib and pazopanib for patients who are cytokine refractory, everolimus for patients who failed VEGFR TKI therapy, and axitinib, which is most recently approved for patients who have received one prior systemic therapy that contained sunitinib, temsirolimus, bevacizumab, or a cytokine [3, 4, 7–13].
Historical treatment with the immunotherapy cytokines interleukin (IL)-2 and interferon (INF)-α remains an option for selected cases of advanced RCC, but has increasingly less clinical implications [4]. These treatments are associated with significant toxicity, in particular IL-2–associated capillary leak syndrome [14, 15].
Radiographically Evident Toxicities Associated with Immunotherapy and Targeted Therapeutic Agents Used in the Treatment of Metastatic Renal Cell Cancer
Interleukin-2. The most frequent and severe complication of IL-2 therapy is the capillary leak syndrome characterized by hypotension, generalized edema, and renal insufficiency [16]. Patients receiving IL-2 therapy may develop pulmonary interstitial edema accompanied by pleural and pericardial effusions within 2–8 days following therapy. This may progress to diffuse air space disease similar to adult respiratory distress syndrome [17, 18]. These findings are detectable on chest radiograph or computed tomography (CT) imaging. This syndrome in most cases is reversible with discontinuation of therapy.
Anti-VEGF therapy: sunitinib, sorafenib, pazopanib, bevacizumab. The most common adverse events associated with the VEGFR TKIs sunitinib, sorafenib, pazopanib, and axitinib include hypertension (HTN), fatigue, hand-foot syndrome (HFS), anorexia, mucositis, diarrhea, hypothyroidism, elevated lipase, metabolic disturbances, and myelosuppression. The most frequent toxicities that occur during treatment with the monoclonal antibody bevacizumab include hypertension, proteinuria, bleeding, thrombosis, and gastrointestinal (GI) perforation [19–21]. Arterial thrombosis has been reported in 3 % of patients treated with pazopanib [8].
Diarrhea or abdominal pain during treatment with sunitinib may be accompanied radiographically by the finding of new bowel wall thickening. Sunitinib-associated enteritis may involve any part of the small bowel, with thickening of the proximal jejunum and mid and distal small bowel observed (Figs. 7.1–7.3).
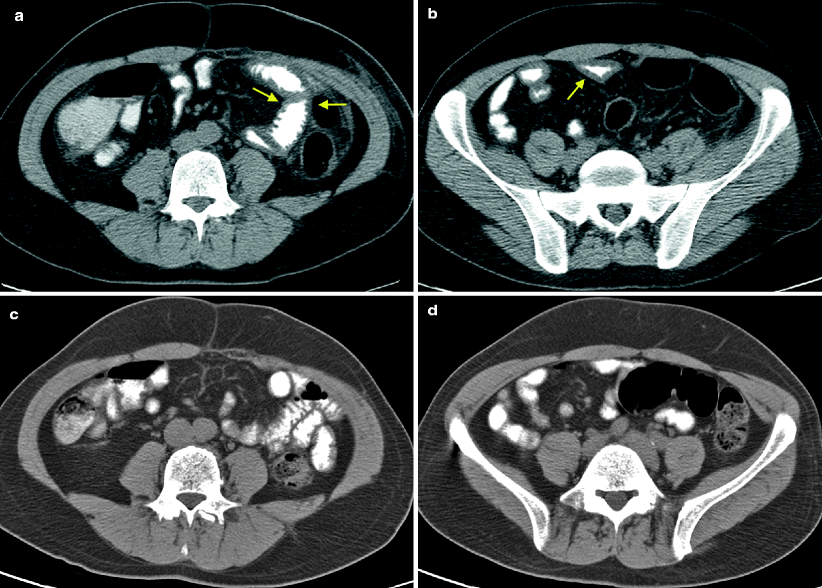
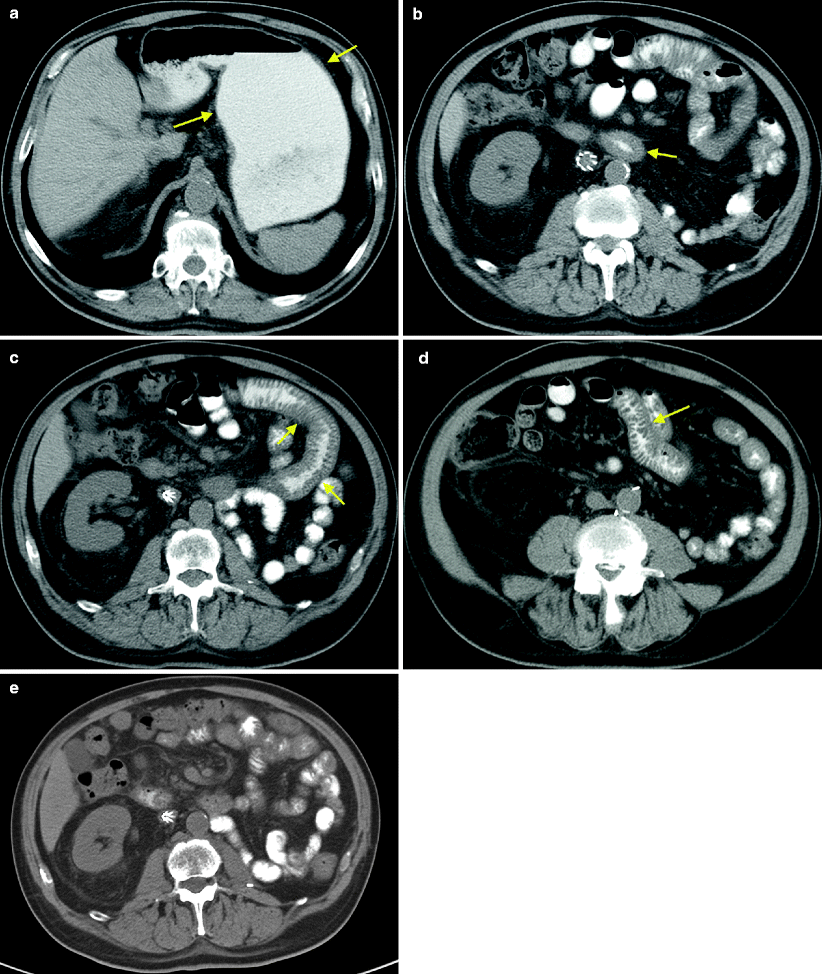
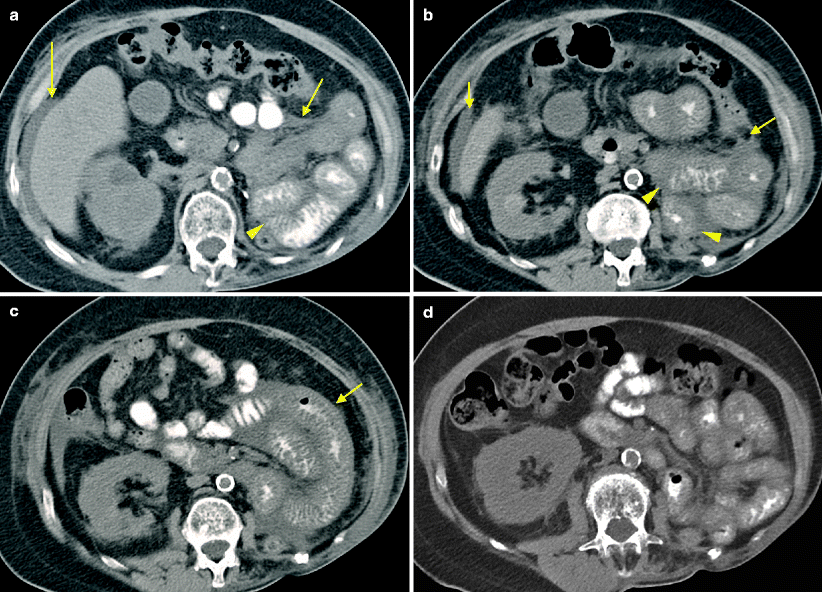

Fig. 7.1
Sunitinib-associated enteritis on CT in the treatment of metastatic RCC. A postnephrectomy patient presented with metastatic recurrence and was started on oral sunitinib at 50 mg daily, 4 weeks on and 2 weeks off. The patient developed grade I mucositis, neuropathy, skin changes, and epistaxis at the end of cycle 1. The patient continued to tolerate therapy with good treatment response. During the treatment break of cycle 7, the patient reported an episode of self-limiting abdominal pain and cramps. 2 weeks later, the routine follow-up CT demonstrated stable disease and a new finding of mild small bowel wall thickening. Representative axial CT images in the mid and lower abdomen demonstrated mild small bowel wall thickening, involving several loops of mid (a, arrows) and distal small bowel (b, arrow). During cycle 8, the patient had another episode of abdominal pain and diarrhea. The patient requested to continue treatment with no reduction in dose. At the end of cycle 9, clinical symptoms and radiographic findings resolved (c, d). He continued to tolerate therapy well with intermittent treatment breaks

Fig. 7.2
Sunitinib-associated enteritis in the treatment of metastatic RCC. A patient with metastatic RCC was started on oral sunitinib 50 mg daily, 4 weeks on and 2 weeks off. The patient complained of grade I nausea during cycle 1 and grade III HFS during cycle 3. A follow-up CT after cycle 4 revealed good treatment response. A repeat CT after cycle 6 showed stable disease and incidental note of small bowel wall thickening. Axial CT images demonstrated mild gastric dilatation (a, arrows), bowel wall thickening of the crossing duodenum (b, arrow), mild bowel wall thickening of the proximal jejunum (c, arrows), and thickening of a distal loop of small bowel (d, arrow). The patient was asymptomatic at the time of imaging, however, during the following treatment cycle, the patient reported several episodes of nausea and vomiting. The patient continued to tolerate therapy at full dose but treatment was suspended for worsening hypertension. Radiographic manifestations of GI toxicity had resolved on follow-up CT (e). Sunitinib was ultimately discontinued due to progression of disease

Fig. 7.3
Sunitinib-associated enteritis in the treatment of metastatic RCC. A patient with metastatic RCC was started on sunitinib with a dosing schedule of 4 weeks on and 2 weeks off. The patient complained of bloating within the first 4 weeks of therapy. Follow-up CT without intravenous contrast obtained at the end of cycle 1 revealed good treatment response, with new small volume ascites and stranding of the mesentery (a, arrows). Bowel wall thickening of the proximal jejunum was attributed to under distention at the time of the initial reading (a, arrowhead). The patient complained of general malaise, fatigue, and abdominal swelling during cycle 2. Repeat CT at the end of cycle 2 revealed stable disease, stable small-volume ascites, mesenteric stranding (b, arrows), and moderate to marked proximal small bowel wall thickening (b, arrowhead). Since bowel wall thickening was a persistent finding, a differential was offered, including edema, hemorrhage, or an infectious or inflammatory process. During the 4 weeks of treatment in cycle 3, the patient noted increasing abdominal girth that had dissipated during the 2 weeks off at the end of cycle 3. CT after cycle 3 revealed persistent small bowel wall thickening (c, arrow). The dose of sunitinib was reduced due to sores on the bottom of his feet. The follow-up CT after cycle 5 demonstrated resolution of ascites and marked improvement in mesenteric infiltration and small bowel wall thickening (d) with clinical improvement of abdominal swelling
Diarrhea occurs in 6 –20 % of patients undergoing sunitinib treatment for RCC [19, 22]. The time to onset of this finding following the initiation of treatment is variable. The frequency of the radiographic manifestation of enteritis has not yet been reported but may be less common than the clinical correlate of abdominal pain, bloating, and diarrhea. In some cases, the radiographic manifestations are not accompanied by concurrent clinical symptoms and therefore deemed “asymptomatic”; in other cases, the CT manifestations of enteritis may precede the clinical symptoms (Fig. 7.2). Drug-induced diarrhea may be managed with dose reduction or interruption but does not typically require discontinuation of treatment [19]. In our experience, clinical and radiographic manifestations of sunitinib-associated enteritis can be self-limited (Fig. 7.1)
Other GI complications potentially encountered include pneumatosis (Fig. 7.4) and hemorrhage (Fig. 7.5). Pneumatosis intestinalis has been reported during certain types of chemotherapy, but of the molecular-targeted therapies it has been described with bevacizumab, an anti-VEGFR agent. Pneumatosis may be asymptomatic, detected first on routine surveillance imaging. Management is conservative in the absence of symptoms of peritonitis [23]
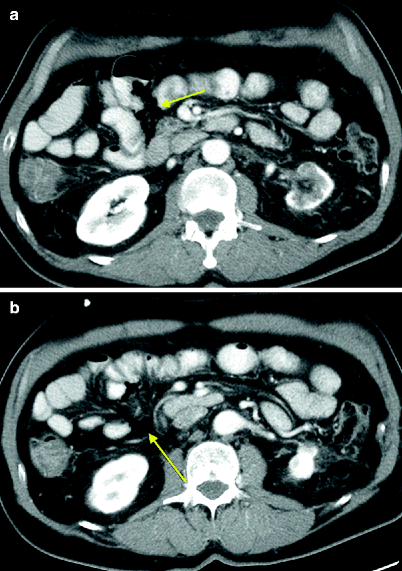
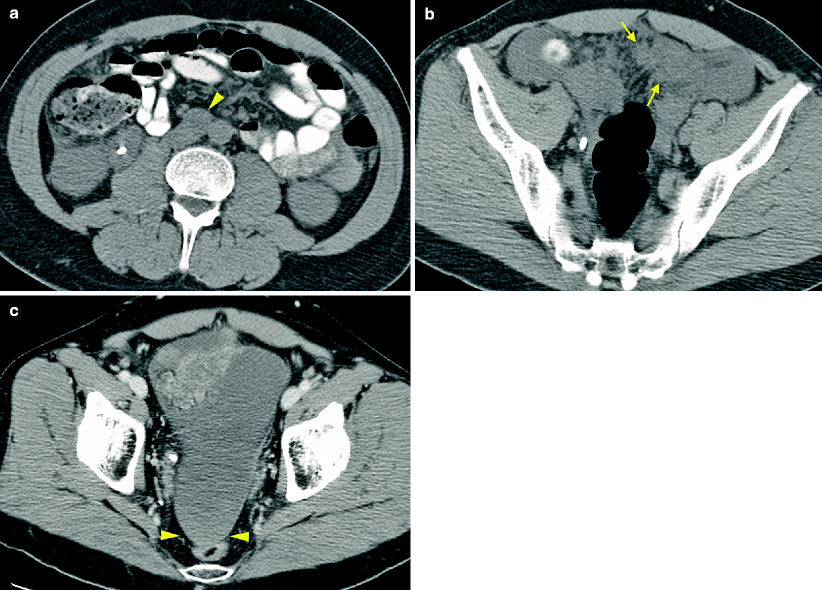

Fig. 7.4
Sunitinib-associated pneumatosis in treatment of metastatic RCC. A patient was randomized to pazopanib on a clinical trial for metastatic RCC. His treatment was changed to sunitinib due to progression of disease. At the end of cycle 1, the patient developed grade III neutropenia. Treatment was held temporarily and he was restarted at a lower dose. Follow-up CT at the end of cycle 3 revealed stable disease with incidental finding of small bowel pneumatosis. CT images reveal discrete and linear arrangement of gas bubbles within the wall of small bowel loops in the mid abdomen consistent with pneumatosis intestinalis (a, arrow). Air within the mesentery is also noted (b, arrow) with no portal venous gas. The patient was asymptomatic. Treatment was temporarily discontinued and restarted at a lower dose with no further problems

Fig. 7.5
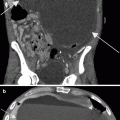
Sunitinib-induced hemorrhage in treatment of metastatic RCC. A patient underwent partial nephrectomy for RCC and was followed with surveillance imaging studies. The patient developed retroperitoneal nodal metastases and was treated with IL-2. Due to subsequent progression of disease, he was enrolled in a clinical trial of sunitinib. A restaging CT scan prior to sunitinib revealed retroperitoneal adenopathy (a, arrowhead) and peritoneal carcinomatosis (b, arrows). At the end of cycle 1, the patient complained of abdominal distention and was found to have a drop in hemoglobin. CT examination revealed increased adenopathy, peritoneal carcinomatosis, and a large amount of high attenuation ascites (HU 40) consistent with hemoperitoneum, considered to be treatment related (c, arrowheads). The dose of sunitinib was reduced. The patient went on to have a mixed response on sunitinib, and was then offered further treatment options
Temsirolimus/everolimus. Toxicities associated with mTOR inhibitors, such as temsirolimus and everolimus, include rash, mucositis, metabolic changes (elevated triglycerides, hyperglycemia, and hypophosphatemia), thrombocytopenia, and nonspecific pneumonitis [19].
Clinical pneumonitis occurs in approximately 15 % of patients treated with everolimus [24]. Patients may present with cough, dyspnea, or both. The incidence of asymptomatic pneumonitis encountered as a new radiographic finding detected during treatment ranges from 39 to 50 % with everolimus and temsirolimus [24, 25]. The high incidence of new pulmonary abnormalities reported during treatment with temsirolimus and everolimus may represent a low-grade drug toxicity that is not clinically detected [24]. The onset of pulmonary findings develops between approximately 3 and 37 weeks after starting treatment [24]. Imaging findings may include patchy ground glass infiltrates and multifocal areas of cryptogenic organizing pneumonitis (COP) with traction bronchiectasis (Figs. 7.6–7.8). The patterns are variable and may change over time. Management options include drug withdrawal, dose reduction, or treatment with steroids [24].
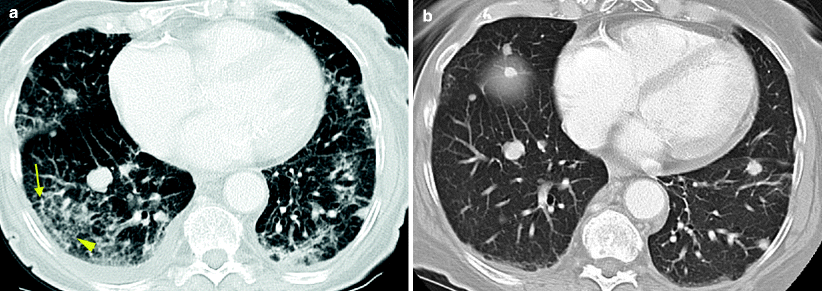
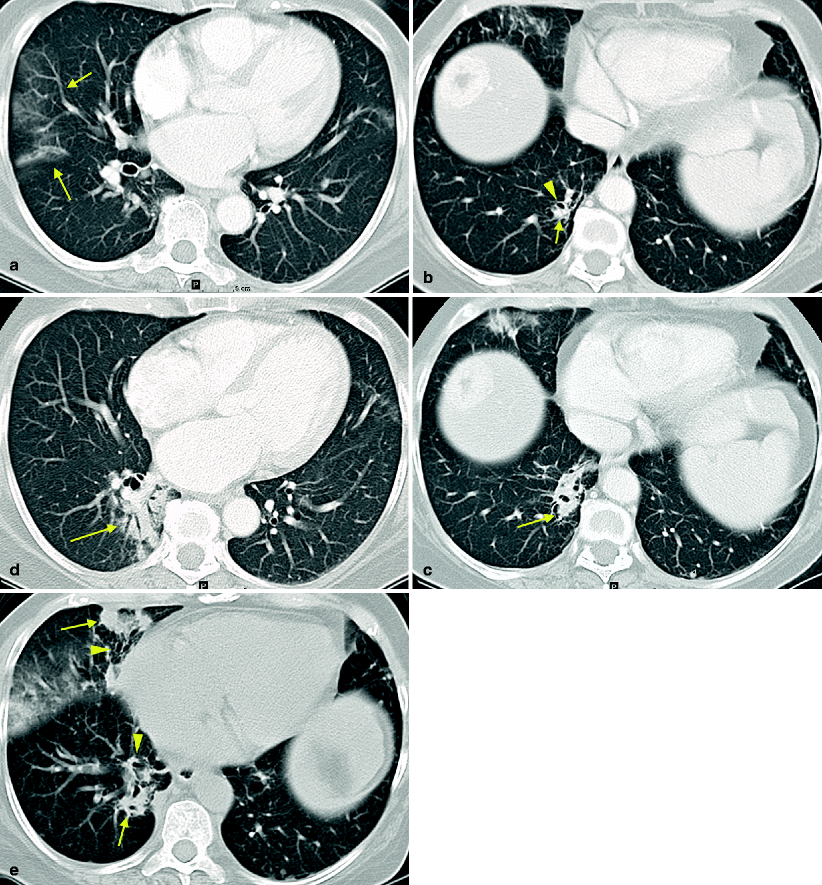

Fig. 7.6
Temsirolimus-associated pneumonitis in treatment of metastatic RCC. A patient presented with pulmonary metastases several years after initial nephrectomy. He was treated with sunitinib followed by sorafenib. Following progression of disease, his treatment was changed to temsirolimus, with the standard weekly dosing schedule. After 4 weeks of temsirolimus, asymptomatic interstitial infiltrates were incidentally noted on a CT performed for treatment evaluation. Axial images of the lower lungs demonstrate new coarse peripheral reticular interstitial thickening (a, arrows) and mild ground glass opacities (a, arrowhead) when compared to baseline CT images prior to initiation of therapy (b). Temsirolimus was eventually discontinued due to leukopenia and thrombocytopenia

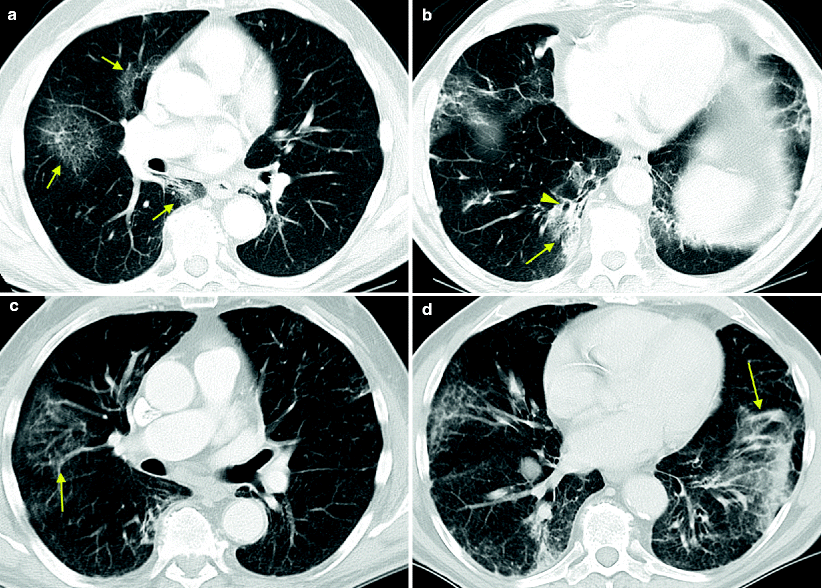
Fig. 7.7
Temsirolimus-associated pneumonitis in treatment of metastatic RCC. A patient was started on temsirolimus after progression of disease while on sunitinib and then on sorafenib. Early in the course of temsirolimus therapy, the patient developed a dry cough that gradually worsened with intermittent low-grade fevers. A chest radiograph revealed “atypical pneumonia.” She continued to have intermittent low-grade fevers on antibiotics. The first CT scan obtained 3 weeks after initiation of treatment revealed multifocal infiltrates. Axial images from two different levels of the lower lungs reveals new ground glass peripheral infiltrate in the right middle lobe (a, arrows) and patchy consolidation (b, arrow) with bronchiectasis (b, arrowhead) in the right lower lobe. CT findings were reported with differential diagnosis of an infectious or inflammatory process. Respiratory symptoms were mild and therapy was continued. Follow-up CT during week 7 demonstrated areas of improvement and areas of worsening consolidation. Axial images from the same levels as in Fig. 7.7a,b revealed improvement in the ground glass infiltrates in the right middle lobe and worsening of COP infiltrates in the right lower lobe with persistent foci of bronchiectasis (c and d, arrow). Follow-up CT during week 13 later revealed increased air-space disease and peripheral COP opacities (e, arrows) with associated bronchiectasis (e, arrowheads) in the anterior right middle and right lower lobes. There was no change in clinical presentation of mild respiratory symptoms. A pulmonary consult determined that the slowly progressive infiltrates associated with mild respiratory symptoms temporally related to initiation of temsirolimus therapy were consistent with drug-induced pneumonitis. A bronchoscopy was performed due to the intermittent fevers and revealed a nonspecific interstitial inflammation and organizing pneumonia without infection. Therapy was held and steroid therapy was initiated with both clinical and radiographic improvement