Fig. 2.1
Radionuclides and radiations
Radiation has unique characteristics in terms of travel and penetrating through other substances. The ability to penetrate varies between radiation types (Fig. 2.1). Alpha and beta particles only travel for a very limited distance. Alpha particles do not penetrate much (travel only a few centimeters in the air) and can be blocked with a single sheet of paper. Beta particles are more penetrating than alpha particles; however, they can be blocked with a few millimeters thick of aluminum sheet. Although they have relatively weak penetrating features, alpha and beta radiations give high amounts of energy to tissues resulting in serious damage if incorporated into the body.
On the other hand, gamma rays are very penetrative and easily travel through thin lead aprons commonly used for protection from X-ray energies used in medical imaging. In Fukushima (especially at the damaged nuclear power plant), there are places with extremely high gamma radiation levels which gamma rays accounted for. A thick concrete block or lead wall is required to block gamma rays. Neutrons are extremely penetrating; they can only be stopped by thick masses of concrete, water, or paraffin. In addition, neutrons have a unique feature of inducing radioactivity when absorbed by stable materials. Neutrons are produced mostly in a specific condition called nuclear fission which takes place inside nuclear reactors in operation or in a nuclear detonation. In the JCO Co. Ltd. Tokai Plant accident, three workers were exposed to very high levels of neutrons which were produced in a nuclear fission induced while they were dissolving U3O8 (18.8 % enriched) and pouring nitrate solution into a precipitation vessel using a funnel. External exposure was the major health problem (IAEA 2000).
The toxicity of a radioactive material depends on the type of radiation emitted, physical and biological half-life, target organs, and toxic natures of the substance itself. Toxicity of radiation is modified by linear energy transfer (LET), which is a measure of how much energy is transferred from ionizing radiation to tissue. Alpha particles and neutrons have high LET, whereas gamma rays and X-rays have low LET. For example, 131I has a short half-life of 8 days. However, 131I has a strong affinity to the thyroid gland, staying in the tissue while emitting beta particles which may result in later development of cancer. Another example is 137Cs that has a relatively long half-life of 30 years; however, the biological half-life is 9 days in small children and approximately 109 days for adults. Cesium acts like potassium, spreading throughout the body after incorporation and is excreted into urine. Plutonium-239 (239Pu) inhaled into the lung moves to the bone and liver, resulting in damage to these organs through the effects of alpha particles over many years. The effects of radiation are broadly divided into two types: external exposure and contamination (Fig. 2.2).
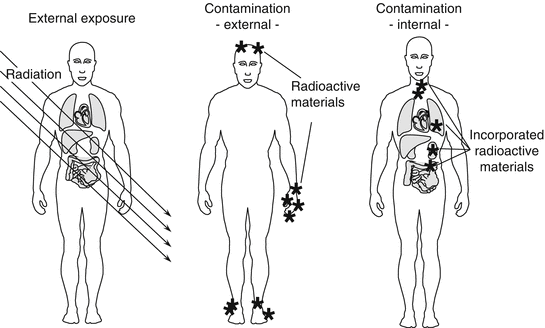

Fig. 2.2
External exposure and contamination
2.5 External Exposure and Contamination
External exposure means being exposed to radiation from the outside of the body. Individuals who are externally exposed to radiation have not absorbed any radioactive substances and are therefore not “radioactive.” In the Fukushima accident, gamma rays emitted from 131I in the early phase and 137Cs in the late phase after the accident were major sources of external exposure.
Contamination takes place internally and/or externally. Internal contamination refers to a status where radioactive materials are incorporated into the body. With internal contamination, the substance may stay in the body for a certain period of time depending on the physical and biological half-life of the substance, and during this time they exert an effect on tissues and organs because of the deposited radiation sources.
The mode of internal contamination is through inhalation, digestion, and absorption from open wounds of the skin. In the Fukushima accident, the highest level of radiation exposure for workers was 678 mSv (Sv: Sievert, unit of irradiation which the body is exposed) of which internal contamination accounted for 590 mSv (The National Diet of Japan 2012). The entry of radioactive materials into the body was mostly through inhalation because it was found later that this worker did not wear effective face masks. The legal radiation exposure for a rescue worker was raised from 100 to 250 mSv on 15 March 2011 (The National Diet of Japan 2012). In the Chernobyl accident, on the other hand, local residents including small children were exposed internally by taking dairy products contaminated with radioactive iodine-131 (131I), resulting in a high incidence of thyroid cancer (The Chernobyl Forum 2006).
External contamination refers to a status where radioactive materials such as radioactive dusts or particulates get attached to the skin or clothes. In this case, the body is exposed to radiation from outside the body, and it carries a high risk of internal contamination by accidental inhalation, ingestion, or absorption of deposited radioactive materials. In the Fukushima accident, most of the severe contamination observed among workers was through exposure to contaminated water. Two workers became contaminated on their feet by immersion in heavily contaminated water in the No. 3 reactor building. Also, radioactive dust or plume released after Fukushima accident contaminated thousands of residents (The National Diet of Japan 2012).
2.6 Biological Effects of Radiation
Living cells undergo divisions vigorously to create new cells. The DNA of the cell has genetic information and is essential for cell division. Beyond a certain level of radiation exposure, the cell membrane and cyto-cellular DNA are damaged. Because genetic material is particularly sensitive to radiation, tissues that divide rapidly (such as bone marrow and intestinal cells) are more sensitive to damage than those with slower cell divisions (such as muscle and neuron) (Fig. 2.3) (IAEA 2002). Natural radiation does exist, to which we are constantly exposed in our daily life. Our body has protective mechanisms to reduce the effects of radiation. However, if the exposure dose of radiation exceeds the levels such that the protective mechanisms do not work anymore, acute radiation effects take place, which is called the deterministic effects (Fig. 2.3).
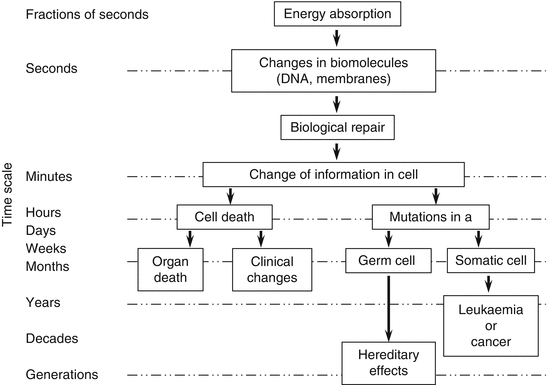

Fig. 2.3
Effects of radiation
Acute effects consist of whole body and local injuries. Acute radiation exposure of the whole body greater than 1 Sv leads to loss of appetite, nausea, vomiting, and diarrhea (these syndromes are called prodromal signs). Radiation injuries to the bone marrow result in pancytopenia and predisposition to bleeding diathesis and infection within 1 or 2 weeks. Gastrointestinal symptoms also ensue following exposure. These symptoms are called acute radiation syndrome (see Chap. 3). Regarding local effects, most of them are dermatological injuries. Although radiation dermatitis is often called radiation burn, the mechanisms involved are quite different from thermal burn. Depending on the different sensitivities of the cells of the dermis to radiation, various pathological changes such as redness (initial erythema), edema, bulla formation, erosion, and ulceration ensue. It takes weeks to develop radiation dermal injuries.
As for long-term effects of radiation, when the DNA of certain tissues is exposed to radiation, cancer can develop as a late effect (Fig. 2.3). In this instance, the DNA injured by radiation is incorporated into the genes as a mutation, resulting in the tissues becoming malignant in the future. This is called the stochastic effect.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





