Genetic syndromes are an infrequently encountered but challenging group of conditions for both pediatric and adult radiologists given the multitude of possible findings and important complications associated with these syndromes. This article reviews characteristic syndromic imaging features, as well as discussing important complications and screening recommendations for a selected group of clinically relevant genetic syndromes affecting both pediatric and adult populations.
Key points
- •
Genetic syndromes are infrequently encountered but challenging group of conditions for both pediatric and adult radiologists.
- •
Both pediatric and adult radiologists should be familiar with characteristic syndromic imaging features and common complications. Such knowledge can assist in image interpretation, guide management, and guide appropriate surveillance.
Introduction
Although genetic syndromes are often thought of as entities falling under the scope of pediatrics and pediatric radiology, the sequelae of these conditions frequently extend into adulthood and are often associated with significant morbidity and mortality. Therefore, both pediatric and adult radiologists should be familiar with characteristic syndromic features and the most common complications, including malignant transformation of benign neoplasms. Such knowledge can assist in image interpretation and help guide management and appropriate surveillance.
Although a complete discussion of every aspect of the included genetic syndromes is beyond the scope of this review article, the overarching goal is to provide an up-to-date review of characteristic imaging findings, management, and screening recommendations for a select group of clinically important genetic syndromes that are encountered in daily clinical practice by general radiologists ( Table 1 ).
| Genetic Syndromes | Key Imaging Features | Current Selected Imaging Screening Recommendations |
|---|---|---|
| NF 1 , | Neurofibromas, CNS gliomas, skeletal involvement (eg, scoliosis, pseudarthrosis), malignant peripheral nerve sheath tumors, neuroendocrine tumors (eg, pheochromocytoma), embryonal rhabdomyosarcoma, breast malignancy |
|
| NF 2 | Schwannomas (eg, bilateral schwannomas involving the vestibulocochlear nerve), meningiomas, spinal lesions (eg, low-grade ependymoma) |
|
| VHL | CNS and retinal hemangioblastomas, pheochromocytomas, renal cysts and RCCs, pancreatic cysts and cystadenomas, pancreatic neuroendocrine tumors, endolymphatic tumors |
|
| TS | Subcortical tubers, subependymal ependymomas, subependymal giant cell astrocytoma, renal angiomyolipoma, RCCs, cardiac rhabdomyomas, pulmonary lymphangioleiomyomatosis |
|
| Multiple endocrine neoplasia syndrome type 1 , | Parathyroid adenomas, pituitary adenomas, pancreatic neuroendocrine tumors, adrenal lesions, carcinoid tumors |
|
| Multiple endocrine neoplasia type 2 | Medullary thyroid carcinoma, pheochromocytoma, parathyroid lesions |
|
| LFS , | Osteosarcoma, soft tissue sarcomas (eg rhabdomyosarcomas), brain tumors (eg astrocytoma, choroid plexus carcinoma), adrenal cortical tumors, breast cancer |
|
| DICER 1 syndrome | Pleuropulmonary blastoma, renal lesions (eg cystic nephroma, Wilms tumor), ovarian sex cord-stromal tumors, genitourinary rhabdomyosarcomas, and pituitary blastoma. Patients also may develop other lesions, such as multinodular goiter |
|
Spectrum of genetic syndromes affecting both children and adults
Neurofibromatosis Type 1
Neurofibromatosis type 1 (NF 1) is a multisystemic neurocutaneous disorder with a broad range of clinical manifestations predominantly involving the skin, orbits, nervous system, and musculoskeletal system. NF 1 is the most common neurocutaneous disorder and one of the most common genetic disorders overall, affecting 1 in 2500 to 3000. There is variable expression of the most common systemic abnormalities in NF 1; however, most patients are diagnosed using major or minor criteria by 1 year of age. The condition is associated with an reduction in life expectancy of 8 to 15 years secondary to associated neoplasms and cardiovascular causes. The NF 1 gene is located on chromosome 17 and produces neurofibromin, a tumor suppressor of the Ras/mitogen-activated protein kinase (MAPK) pathway that, when mutated, predisposes to tumor development in both pediatric and adult populations. The inheritance pattern is considered autosomal dominant; however, the condition occurs sporadically in up to 50% of cases.
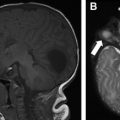
Neurofibromas are benign peripheral nerve sheath tumors classified as plexiform neurofibromas, localized neurofibromas, and diffuse cutaneous neurofibromas. Localized neurofibromas are not specific to NF 1, and diffuse cutaneous neurofibromas are not typically a radiologic diagnosis. Plexiform neurofibromas are frequently seen in patients with NF 1, involve long segments of nerves typically extending into adjacent soft tissues, and present clinically as a so-called bag-of-worms subcutaneous mass. On plain radiography, these lesions are seen as a nonspecific soft tissue mass without calcification. If the lesions are large enough, bony remodeling can be seen secondary to mass effect. On computed tomography (CT), the lesions are typically low density. Magnetic resonance (MR) imaging is the recommended modality for more comprehensive evaluation, and lesions are typically T1 hypointense, T2 hyperintense, and show variable postgadolinium enhancement patterns. On T2-weighted images, these lesions may show the target sign characterized by peripheral hyperintensity and central hypointensity. Of particular concern is the potential for plexiform neurofibromas to undergo malignant degeneration into malignant peripheral nerve sheath tumors (MPNSTs). MPNSTs are aggressive lesions that frequently metastasize. Malignant degeneration occurs in approximately 5% of patients with NF 1. Although differentiation of benign plexiform neurofibromas and MPNSTs is difficult from imaging alone, lesions that are symptomatic show substantial interval growth, show cystic degeneration/necrosis, or have infiltrative margins should alert the reader of the possibility of malignant degeneration ( Fig. 1 ). , 18F-fluorodeoxyglucose (FDG) PET can be helpful because MPNST typically shows increased FDG avidity.

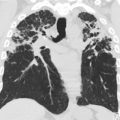
NF 1 is associated with several central nervous system (CNS) neoplasms, such as optic pathway gliomas and brain stem gliomas. Optic glioma affects 15% to 20% of patients with NF 1 and represents one of the diagnostic criteria of NF 1 ( Fig. 2 ). These lesions are typically considered low-grade lesions (World Health Organization grade I). A common diagnostic dilemma in patients with NF 1 is the classically described unidentified neurofibromatosis objects (UNOs), which are T2 hyperintense and T1 isointense and are most commonly seen in the globi pallidi. Depending on location, these lesions may or may not resolve with age. Persistent UNO lesions may mimic CNS neoplasms associated with NF 1, such as a brainstem glioma. In such situations, MR spectroscopy may assist the reader in making such a distinction.

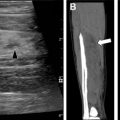
Skeletal abnormalities in patients with NF 1 can often be described using plain radiography and include characteristic findings of sphenoid wing dysplasia, scoliosis, neuroforaminal widening, pseudoarthrosis of the tibia, and rib notching ( Fig. 3 ). , Because these changes progress over time, skeletal involvement of NF 1 may cause substantial morbidity in adulthood. Patients are also at risk for vertebral degenerative changes, dural ectasia, and spinal compression as well as osteoporosis, which typically occurs at an earlier age.

NF 1 is associated with increased incidence of several neoplasms outside of the CNS, including embryonal rhabdomyosarcoma, juvenile myelomonocytic leukemia, and neuroendocrine tumors. Despite their increased incidence relative to the general population, the overall incidence remains low. For example, the incidence of embryonal rhabdomyosarcoma is approximately 1% in patients with NF 1. As such, routine asymptomatic imaging of pediatric patients with NF 1 is not currently recommended. However, clinicians may consider whole-body MR imaging between the ages of 16 and 20 years to assess internal tumor burden and assist with long-term care planning.
Adult patients with NF 1 are at risk for malignancies related to NF 1. For example, NF-associated pheochromocytomas are more commonly diagnosed in adult patients with NF 1, with a median age at diagnosis of 43 years. Adult women with NF 1 are at increased risk of breast cancer, especially before the age of 40 years, and show significantly poorer 5-year survival. The National Comprehensive Cancer Network guidelines recommend an annual mammogram starting at age 30 years and consideration of contrast-enhanced breast MR imaging for female patients with NF 1.
Neurofibromatosis Type 2
Neurofibromatosis type 2 (NF 2) is an autosomal dominant neurocutaneous disorder unrelated to NF 1 or neurofibromas. NF 2 is characterized by the development of multiple CNS lesions, including intracranial schwannomas, meningiomas, and spinal lesions such as low-grade ependymomas. NF 2 typically presents in young adults aged 18 to 24 years with an incidence of 1 in 50,000. The NF 2 gene is located on chromosome 22 and encodes the merlin protein, which plays a role in tumor suppression in neuronal cells, Schwann cells, and meningeal cells.
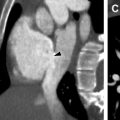
NF 2 presents differently in pediatric and adult populations. The Manchester diagnostic criteria are one of several diagnostic tools that have evolved over time to become more sensitive in diagnosing NF2 in typical pediatric and adult population presentations. The classic adult presentation of NF 2 consists of bilateral schwannomas involving the vestibulocochlear nerve with clinical presentations of hearing difficulties, tinnitus, and balance issues ( Fig. 4 ). Schwannomas also frequently involve spinal and peripheral nerves. Meningiomas may occur throughout the CNS, and intracranial meningiomas are associated with increased mortality in patients with NF 2. Intraspinal ependymomas are typically low grade and indolent. MR imaging is the imaging modality of choice in the evaluation of NF 2–related CNS lesions. In the evaluation of vestibular schwannomas, readers should turn their attention to the internal auditory canal/cerebellar pontine angle, where lesions are typically T1 hypointense to isointense, T2 hyperintense, and avidly and homogeneously enhancing on postcontrast MR images. Meningiomas are typically T1 isointense or hypointense and diffusely enhancing with a dural tail. T2 bright perilesional edema and calcification seen as blooming on T2* gradient recalled echo may be encountered. Indistinct and infiltrating margins with restricted diffusion can signal atypical and malignant meningiomas. Ependymoma presents as an expansile cord lesion that is T1 isointense or hypointense, T2 bright, and avidly enhancing. A T2 dark peripheral rim represents associated hemorrhage and is seen in up to 33% of cases (the cap sign).

Pediatric patients with NF 2 often do not present with bilateral cranial nerve 8 schwannomas but with symptoms related to a nonspecific neuropathy, an apparently isolated meningioma, an extracranial schwannoma, or ocular symptoms. Pediatric patients with NF 2 initially presenting with nonclassic lesions may harbor severe multitumor disease. Baser and colleagues showed that the risk of mortality increases with younger age at diagnosis.
Evans and colleagues recommend MR screening for neoplasms in patients with family history of NF 2 or those presenting with a unilateral vestibular schwannoma or other type of schwannoma, meningioma, or retinal hamartoma. For those patients in whom the diagnosis of NF2 is established, the investigators recommend annual or biannual brain MR imaging starting at 10 years using a high-resolution technique (slice thickness 1–3 mm) with coverage of the internal acoustic meatus in at least 2 planes. Because CNS lesions associated with NF2 are avidly enhancing, gadolinium administration is recommended if clinically appropriate. Imaging of the spine should be obtained starting at 10 years and repeated at intervals of 24 to 36 months, preferably with contrast. If NF 2–associated lesions are detected on either brain or spinal imaging, the next repeat examination should occur 6 months later to assess rate of growth. As patients enter into adulthood, annual MR imaging of the brain is still recommended; however, the interval of spinal imaging can be extended to 3 to 5 years.
Von Hippel-Lindau Syndrome
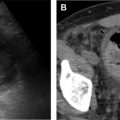
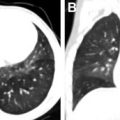
Von Hippel-Lindau syndrome (VHL) is a rare, autosomal dominant syndrome with incidence of 1 in 35,000 to 50,000. VHL is characterized by a germline mutation resulting in inactivation of the VHL tumor suppressor gene on chromosome 3. This mutation has variable expression and can result in dozens of both benign and malignantneoplasms affecting more than 10 organs. The most common lesions include CNS and retinal hemangioblastomas (HBs), pheochromocytomas, renal cysts and renal cell carcinoma (RCC), pancreatic cysts, pancreatic cystadenomas, pancreatic neuroendocrine tumors, endolymphatic sac tumors, and epididymal cystadenomas ( Figs. 5 and 6 ). Although most of the lesions associated with VHL are treatable, affected patients experience increased morbidity and mortality. The most common causes of death in patients with VHL are RCC and complications resulting from cerebellar hemangioblastomas. VHL shows high penetrance, with 90% of patients diagnosed with a tumor by age 65 years and a mean age of 26 years at first tumor detection.


The most characteristic tumor in VHL is the CNS hemangioblastoma, which can occur in the cerebellum, brainstem, spinal cord, and less frequently in the supratentorial brain ( Fig. 7 ). Of all locations, spinal cord HBs are most specific to VHL. CNS HBs are reported in 60% to 80% of patients with VHL, with 44% to 72% of these lesions found in the cerebellum. The mean age at diagnosis is 33 years, with symptoms including headaches and ataxia. HBs are often multifocal and, if a cerebellar HB is detected, imaging of the spinal cord is recommended because coexisting lesions are commonly seen. HBs are typically characterized on imaging as having both solid nodular and cystic components, although cystic components are not always seen. CT shows a low-density cyst and an isodense/hyperdense nodule that enhances avidly after contrast administration. MR imaging findings are similar, with the nodule isointense to brain on T1-weighted images and avidly enhancing after gadolinium administration with both the nodule and cyst hyperintense on T2-weighted images. Development of new cystic components or enlargement of existing cystic components of an HB often correlate with worsening symptoms, especially when seen with peritumoral edema, and may redirect from expectant management to surgical resection.