Genital Tract—CT, MR, and Radiographic Imaging
William E. Brant
Female Genital Tract
The primary modality for imaging of the female genital tract is US using transabdominal, transvaginal, and Doppler techniques. Sonography of the genital tract is reviewed in Chapter 36. MR and CT are used to stage and follow up pelvic malignancies and to supplement US by providing additional characterization of lesions (1). MR, because of its excellent capacity to differentiate tissue types, is particularly useful in making an imaging diagnosis of pelvic disease. Diffusion-weighted MR has the potential to aid in the discrimination between benign and malignant lesions and to provide improved detection of peritoneal metastases and tumor recurrence (2,3). MDCT with isotropic voxel acquisition allows for multiplanar reformatted images of high quality to improve the recognition of anatomic variants and complex pathology (4). In addition, many uterine and adnexal lesions may be discovered incidentally by pelvic CT or MR performed for other reasons (5). Hysterosalpingography (HSG) is combined with US, CT, and MR to diagnose congenital anomalies of the female genital tract and mechanical causes of infertility (6). The HSG is performed by cannulating the cervix and injecting a contrast agent into the cavity of the uterus and fallopian tubes. Free communication of these lumina with the peritoneal cavity is evidenced by the free spill of the contrast agent into the peritoneal cavity outlining loops of bowel. Sonohysterography is an alternative to HSG. Isotonic saline is injected into the uterine cavity, whereas the uterus is examined sonographically (7). Virtual HSG is an emerging MDCT technique that offers the potential of high-resolution images depicting both the internal and external surfaces of the uterus and fallopian tubes (8).
Anatomy
The uterus is a pear-shaped muscular organ located between the bladder and rectum. The anterior and posterior surfaces of the uterus are covered by peritoneum, the folds of which extend laterally to the pelvic sidewalls forming the broad ligament. Peritoneum reflecting off the uterus and the bladder forms a shallow anterior vesicouterine pouch. A “bare area” of extraperitoneal space is present between the lower uterus and bladder. This is an important area for the direct spread of tumor from one organ to the other. Posteriorly, the peritoneum reflects onto the rectum and forms a deep recto-uterine pouch or cul-de-sac. The peritoneum completely covers the uterus and the posterior vaginal fornix. Only the thin wall of the vagina separates the vaginal cavity from the cul-de-sac, allowing transvaginal access to the intraperitoneal space for US-guided culdocentesis or biopsy. The uterus, cervix, and the upper one-third of the vagina are derived from the Müllerian ducts, whereas the lower two-thirds of the vagina arise from the urogenital sinus. Parametrium refers to the connective tissue adjacent to the uterus between the folds of the broad ligament and adjacent to the vagina. Uterine vessels and lymphatics pass through the parametrium. The broad ligament covers the fallopian tubes hanging over them like a sheet folded on a clothesline enveloping the vessels of the parametrium. The broad ligament is well outlined when fluid is present in the pelvic peritoneal cavity. The fundus of the uterus is that portion that extends cephalad from the origin of the fallopian tubes. The body extends from the fallopian tubes to the isthmus, a slight constriction that marks the location of the internal cervical os. The cervix is cylindrical in shape and is 3 to 4 cm in length. Its lower portion, including the external os, protrudes into the vagina and is surrounded by the vaginal fornices. The ureters pass 2 cm lateral to the supravaginal portion of the cervix. The vagina is a muscular tube that is a flattened oval shape on cross-sectional images. The urethra is a prominent tubular structure that courses in the anterior wall of the vagina (9).
Ovaries vary in size and appearance depending on the woman’s age, hormonal status, and stage of the menstrual cycle (10). The adult ovary is oval with a maximal dimension of 5 × 3 × 2 cm. Abnormalities of size are best determined by calculating ovarian volume using the formula (length × width × thickness × 0.52). Maximum ovarian volume is 9 cc before menarche, 22 cc in menstruating women, and 6 cc in postmenopausal women. The location of the ovaries is variable in different patients and even in the same patient at different times depending on the degree of bladder filling and the presence and size of other structures in the pelvis. The typical location is lateral, superior, or posterior to the uterine fundus, or in the cul-de-sac. When the uterus is retroverted, the ovaries are anterior or lateral to the uterus. The pelvic ureters form an important anatomic landmark that assist in the recognition of
the origin of pelvic masses (10). The ovaries are anterior to the ureters, so an ovarian mass will displace the ureter posteriorly or posterolaterally. Iliac lymph nodes are lateral to ureters, so adenopathy will displace the ureters medially or anteromedially.
the origin of pelvic masses (10). The ovaries are anterior to the ureters, so an ovarian mass will displace the ureter posteriorly or posterolaterally. Iliac lymph nodes are lateral to ureters, so adenopathy will displace the ureters medially or anteromedially.
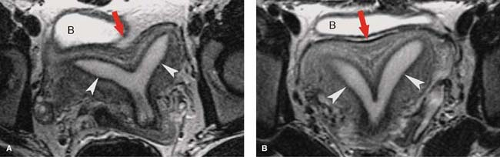
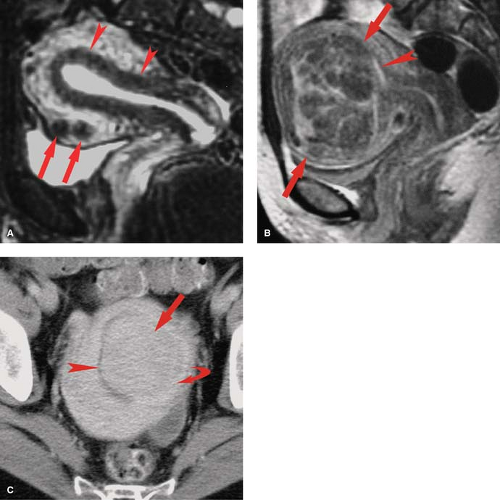
Normal MR Anatomy. The internal anatomy of the uterus is depicted best on T2WI. On T2WI, the endometrium appears as a high-signal intensity central stripe surrounded by the low-signal intensity junctional zone myometrium (Fig. 34.1). The endometrium may normally be up to 14 mm in thickness in women of menstrual age. The bulk of the myometrium is intermediate signal intensity. The low-signal intensity of inner junctional zone of the myometrium on T2WI is due to lower water content. On T1WI, the entire uterus is low in signal intensity and the internal anatomy of the uterus is poorly demonstrated. With gadolinium enhancement, uterine zonal anatomy becomes evident on T1WI. The cervix is largely composed of collagenous tissues that are low in signal intensity on both T1WI and T2WI, providing a dark background for visualization of hyperintense cervical carcinomas. The endocervical epithelium and mucus is homogeneous high signal on T2WI. High-resolution MR using surface or intravaginal coils shows two zones in the cervical fibromuscular stroma, a darker inner zone contiguous with the uterine junctional zone and an intermediate signal outer zone distinctly darker than the myometrium. Vaginal anatomy is also best seen on T2WI, which shows the muscular vaginal wall as low in signal with the epithelium and mucus as high in signal. Aqueous vaginal gel may be inserted for MR scanning to distend the vagina and optimize the evaluation of the vagina and cervix (11). The normal ovaries of fertile women are easily identified by the bright signal of the follicles on T2WI (Fig. 34.2). The follicles are low or intermediate in signal on T1WI. The cortex of the ovary in the premenopausal woman is darker in the signal than the medulla on T2WI. The postmenopausal ovary is more difficult to identify because of the absence of follicles and the cortex and medulla being nearly equal in signal on both T1WI and T2WI.
MR is sensitive to physiological changes that affect the uterus and ovary during the menstrual cycle (12). Signal intensity of the myometrium is the highest during late proliferative and early secretory phases and is the lowest during menstruation and early proliferative phase. Low-intensity myometrial lesions such as leiomyomas and adenomyomas are best demonstrated when the myometrium has the highest signal intensity in mid-menstrual cycle. The ovaries vary in size and appearance during the menstrual cycle and are largest with a dominant follicle just prior to ovulation.
Normal CT Anatomy. Because the position of the uterus is so variable on axial plane CT, the outline of the uterus often appears lobulated or bulbous solely because of the position (Fig. 34.1). The uterus is uniform in soft-tissue attenuation, and its internal anatomy is not well demonstrated by unenhanced CT. Because the myometrium is highly vascular, the uterus enhances more than most other pelvic organs. Fluid in the uterine cavity is usually of low density. The ovaries are easily mistaken for unopacified bowel loops in the pelvis. Ovarian follicles are recognized by their fluid attenuation (Fig. 34.2) (13). The vagina is seen in the cross-section as a flattened ellipse of soft-tissue density between the bladder and rectum. Normal fallopian tubes are usually not evident on CT. Multiplanar reformatted MDCT images are of great value in the interpretation of complex pelvic anatomy and pathology (4).
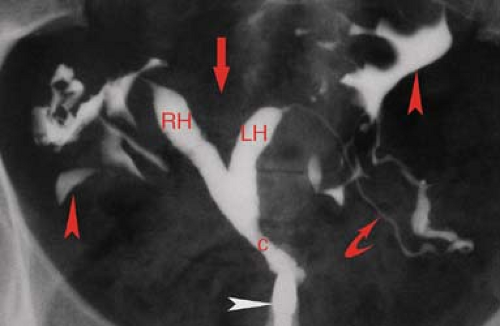
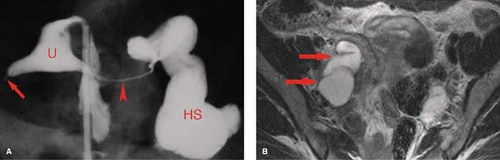
HSG is primarily used for the evaluation of infertility to demonstrate the morphology and patency of the uterine canal and fallopian tubes (Fig. 34.3). Contrast injected into the uterine cavity outlines the endocervical canal, uterine cavity, and lumen of the fallopian tubes with free spill of contrast into the peritoneal cavity in the normal patient (6). The uterine cavity is sharply defined and triangular in shape with normal mild concavity in the fundal region. The size of the cavity varies with parity. The endocervical canal is cylindrical in shape, 3 to 4 cm in length and 1 to 3 cm in width. Folds in the endocervical mucosa form a normal serrated appearance. The normal fallopian tubes are 10 to 12 cm in length, extending from the cornua of the uterus. The lumen is thread like (1 to 2 mm) until it reaches the ampulla where it expands to 5 to 10 mm and rugal folds become visible. Patency of the tubes is confirmed by the dispersal of contrast within the peritoneal cavity outlining the loops of bowel.
Congenital Anomalies
Congenital anomalies of the female genital tract are a common cause of infertility, seen in up to 9% of women evaluated for infertility or repeated abortion. In addition, unrecognized anomalies may be mistaken for other types of pathology, such as leiomyoma (14). Most anomalies result from arrested development or incomplete fusion of the paired Müllerian duct that forms the uterus, cervix, and fallopian tubes (15). Urinary tract abnormalities are found in 20% to 50% of patients with uterine anomalies. Arrested Müllerian duct development may result in uterine aplasia or unicornuate uterus with a single fallopian tube. Ipsilateral renal agenesis is found in 5% to 20% of patients with these anomalies. Failure of complete fusion of the Müllerian duct results in varying degrees of duplication, from uterus didelphys, with two uteri, two cervices, and two vaginas; to bicornuate uterus with two uterine horns, one (unicollis) or two (bicollis) cervices, and one vagina; to an arcuate (septate) uterus with a midline septum dividing the uterus into two cavities (Figs. 34.3, 34.4). Uterine anomalies should be suspected when the uterus appears abnormal in size, contour, or position. The classification of the
anomaly is made by a combination of physical examination and MR examination. HSG is used to demonstrate the uterine cavity and fallopian tubes. Classification of Müllerian duct anomalies is made by the American Society of Reproductive Medicine (14,16).
anomaly is made by a combination of physical examination and MR examination. HSG is used to demonstrate the uterine cavity and fallopian tubes. Classification of Müllerian duct anomalies is made by the American Society of Reproductive Medicine (14,16).
Benign Conditions
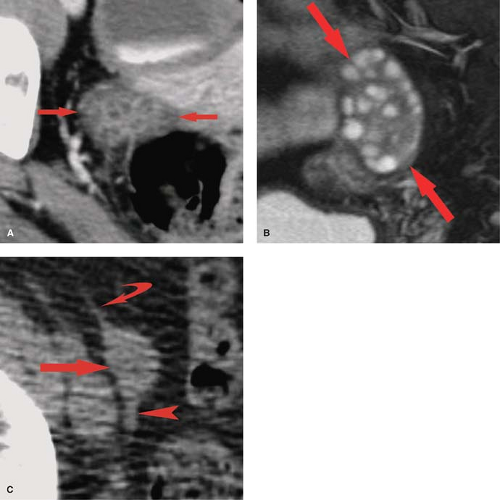
Leiomyomas are the most common uterine tumor affecting 50% of women of reproductive age. Most women are asymptomatic, but the tumors may cause excessive bleeding, pelvic pain, mass symptoms, and infertility. Tumors are benign and made up of smooth muscle and a variable amount of fibrous tissue. Tumors with scant fibrous tissue enhance brightly, whereas those with abundant fibrous tissue enhance poorly. Most tumors are intramural (within the myometrial wall), whereas others are submucosal (beneath the endometrium) or subserosal (beneath the serosa). Subserosal or submucosal tumors may be pedunculated and on long stalks. Submucosal tumors are prone to ulcerate, resulting in severe menorrhagia. MR provides the best characterization of size, number, and location (17). Leiomyomas are usually low signal compared to myometrium on both T1WI and T2WI, although visualization is best on T2WI (Fig. 34.5). Areas of degeneration and cystic change cause inhomogeneous high internal signal. The tumors are well-demarcated from adjacent myometrium by a discrete rim of low signal. Contrast enhancement does not improve leiomyoma detection or characterization. On CT, leiomyomas appear as homogeneous or heterogeneous masses that may be hypodense, isodense, or hyperdense relative to enhanced myometrium. Coarse calcifications within the mass are common and characteristic (Fig. 34.6). Cystic degeneration produces interior low density. Diffuse enlargement of the uterus and lobulation of its contour are common. Pedunculated leiomyomas may appear as adnexal rather than uterine masses. Lipoleiomyomas contain macroscopic fat detected on CT (Fig. 34.7), or by MR using fat-suppression sequences.
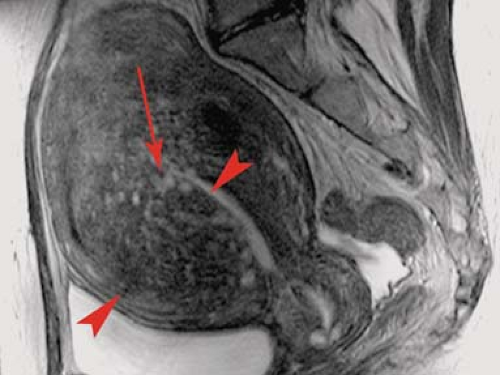
Adenomyosis is a benign disease of the uterus characterized by the presence of ectopic endometrial glands and stroma within the myometrium, eliciting surrounding myometrial hypertrophy (18). Patients present with dysmenorrhea or menorrhagia. The disease may be focal or diffuse. MR provides the best detection of the disease. Diffuse disease is indicated by regular or irregular thickening of the junctional zone myometrium greater than 12 mm. The low-signal abnormality corresponds to myometrial hypertrophy. Half of the patients also demonstrate high-signal foci within the myometrium corresponding to islands of endometrial glands with cystic change or hemorrhage (Fig. 34.8). Focal disease is evidenced by low-signal masses within the myometrium on T2WI. These masses are isointense to myometrium on T1WI. High-signal foci occasionally seen on T1WI represent hemorrhage. Differentiation from leiomyomas is difficult. Leiomyomas are characteristically well circumscribed, whereas adenomyomas are poorly defined with vague margination. Adenomyosis is not routinely evident on CT. US findings are usually subtle and nonspecific.
Nabothian cysts are retention cysts of the mucous-secreting glands of the cervical epithelium. They are common, benign, and generally of no clinical significance. On MR, they appear as bright, round, well-defined structures in the cervix on T2WI (see Fig. 34.1A). On T1WI, they are isointense to urine or muscle. Small-size and sharp margins differentiate nabothian cysts from adenoma malignum, a multicystic form of adenocarcinoma of the cervix. This malignancy appears as multicystic mass, with numerous small cysts on a background of enhancing solid tissue (19).
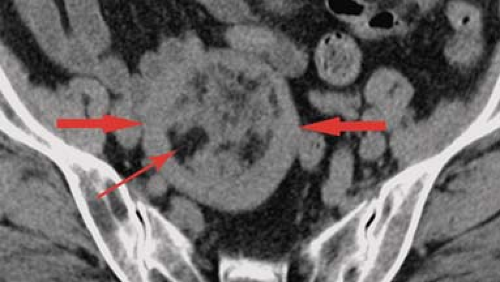
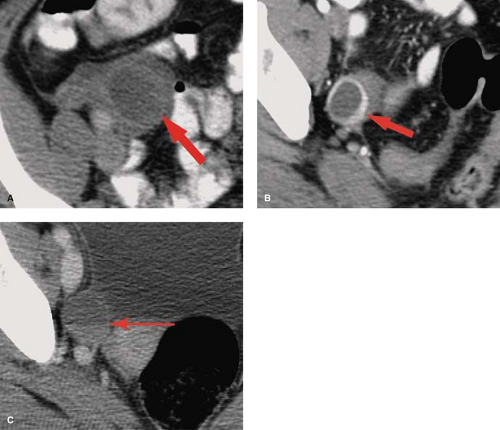
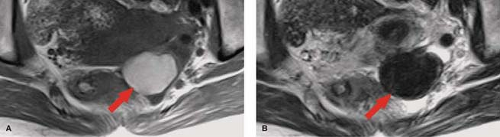
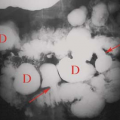
Physiological ovarian cysts and normal ovarian follicles contain simple fluid that is low signal on T1WI and high signal on T2WI. A uniform, thin, dark wall is evident on T2WI. Gadolinium enhancement of the cyst wall is common but not constant. On CT, they are well defined, thin walled, and have homogeneous internal density near water. Size less than 3 cm is indicative of physiological ovarian follicle (Fig. 34.9) (20). The corpus luteum is a normal physiologic ovarian structure that develops at the site of the dominant follicle following ovulation. A normal corpus luteum is smaller than 3 cm, has a diffusely thick wall, and has a prominent peripheral blood flow. A crenulated, collapsed cyst appearance is usual (21). Central hemorrhage is often present (1).
Hemorrhagic functional ovarian cysts appear high signal on T1WI if a large amount of methemoglobin is present. If predominantly intact red blood cells are present, the cyst appears low signal on T2WI. Thus hemorrhagic cysts may be low signal on both T1WI and T2WI, high signal on T1WI and low signal on T2WI, or low signal on T1WI and high signal on T2WI. Layering of blood products may be present. The absence of gadolinium enhancement differentiates internal blood clot adherent to the cyst wall from a solid nodule. On CT, hemorrhagic cysts appear as thin-walled cysts with internal density near water or higher in attenuation depending on the physical state of the blood products (Fig. 34.9). Atypical
cysts can be followed with US to determine if they resolve after one or two menstrual cycles.
cysts can be followed with US to determine if they resolve after one or two menstrual cycles.
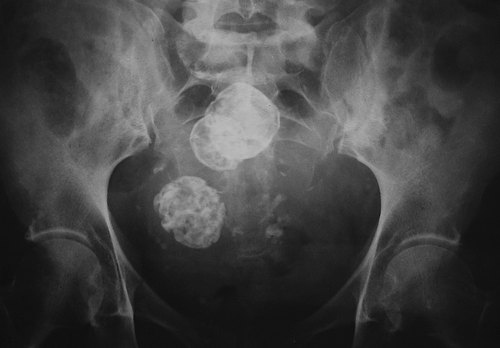 Figure 34.6. Leiomyoma Calcifications. Conventional radiograph of the pelvis reveals multiple leiomyomas with characteristic “popcorn” calcifications. |
Endometriosis is the presence of endometrial tissue in locations outside of the uterus (22). The endometrial implants respond to cyclic hormonal stimulation resulting in recurrent bleeding, inflammation, and fibrosis. Hallmarks of disease include numerous tiny implantations of endometrial tissue on peritoneal surfaces, development of endometriomas (endometrial cysts filled with hemorrhage), and formation of adhesions between surrounding tissues. The most common sites of involvement are the ovaries, the cul-de-sac, and peritoneal reflections over the uterus, fallopian tubes, bladder, and rectosigmoid colon. All imaging modalities have high sensitivity for detection of endometriomas, but all lack the ability to reliably detect the tiny endometrial implants, which are commonly smaller than 3 mm. Endometriomas (“chocolate cysts”) contain blood products of various ages reflecting recurrent episodes of bleeding corresponding to the menstrual cycle. They are characteristically multiple, bilateral, and located in the cul-de-sac. MR shows the cysts to be homogeneous high intensity on T1WI and characteristically low signal on T2WI, a finding termed “T2 shading” (Fig. 34.10). Loss of signal on T2WI is caused by the presence of methemoglobin within the cysts. Iron concentration and viscosity increase within the cysts as water is resorbed. The “shaded” fluid may layer dependently within the cyst. Cysts may appear heterogeneous because of the varying age of contained blood products. The cyst wall is usually low signal representing fibrous tissue or hemosiderin. Fat-saturation T1WI improves visualization of small implants on peritoneal surfaces. On CT, endometriomas appear as complex cystic pelvic masses, frequently with relatively high attenuation fluid components. Inflammation and fibrosis are prominent. Multiple pelvic organs may be incorporated into a mass. Hydrosalpinx is a common associated finding
(30%) (23). Endometriosis may involve the bowel or the urinary tract or may occur outside the pelvis and in surgical scars (24). Malignant transformation of endometriosis is a rare occurrence.
(30%) (23). Endometriosis may involve the bowel or the urinary tract or may occur outside the pelvis and in surgical scars (24). Malignant transformation of endometriosis is a rare occurrence.
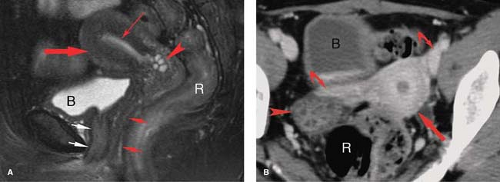
Hydrosalpinx is a common adnexal mass that may occur as an isolated lesion or as a component of a complex adnexal mass (23). Occlusion of the fallopian tube, caused by infection, surgery, tumor, or endometriosis, results in fluid accumulation and dilatation of the tube. The most common cause is pelvic infection. Imaging of isolated hydrosalpinx demonstrates a sausage-, C-, or S-shaped adnexal structure distended with fluid of variable character that may be serous fluid, hemorrhage (hematosalpinx), or pus (pyosalpinx) (Fig. 34.11). Tortuosity, dilatation, and folding of the tube may resemble an ovarian tumor. Multiplanar images and identification of a normal ovary on the ipsilateral side assist in confirming the diagnosis. MR is sensitive to the nature of the fluid within the tube showing low signal on T1WI with high signal on T2WI for simple serous fluid. Proteinaceous fluid, hemorrhage, or pus cause high signal on T1WI. Pelvic inflammatory disease, endometriosis, or fallopian tumor may incorporate hydrosalpinx as a component of a complex cystic and solid adnexal mass. Hydrosalpinx is a common finding on HSG performed for infertility (Fig. 34.11A).
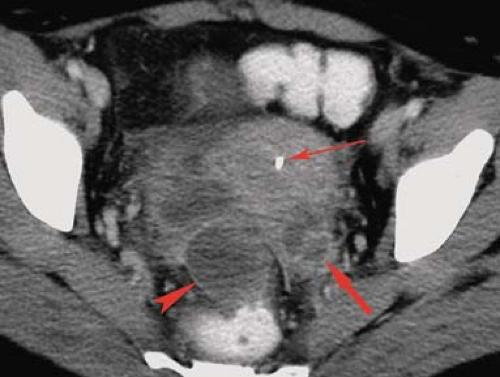
Pelvic inflammatory disease is a common affliction of women of reproductive age. The usual causative organisms are a mixture of anaerobic and aerobic bacteria from the vagina. Uncommon organisms include actinomycosis and tuberculosis (25). Endometritis and myometritis are treated medically. Imaging is performed to detect tubo-ovarian abscess and pyosalpinx, complications that may require surgical treatment. Early findings include pelvic and edema and stranding in the parametrium and paraovarian tissues. Pyosalpinx appears as a thick-walled edematous tube that contains complex fluid (23). Tubo-ovarian abscess appears as a thick-walled fluid-filled adnexal mass that incorporates the ovary and commonly a dilated fallopian tube (Fig. 34.12) (26). Gas bubbles are occasionally present within the collection and are highly indicative of abscess. Adenopathy and ascites may be present.
Peritoneal inclusion cyst is an increasingly common and difficult-to-treat cause of chronic pelvic pain (27). Adhesions from previous surgery or inflammatory process entrap the ovary within a fluid collection that extends into peritoneal recesses. Continuing secretion of fluid by the active ovary is not absorbed by the diseased peritoneal surfaces, producing pain and pressure systems. Imaging shows a fluid collection that includes the ovary. The fluid is usually simple and characteristically extends into peritoneal recesses, giving the collection an angled or pointed rather than spherical or oval shape (Fig. 34.13). Effective treatment generally requires removal of the ovary.
Benign cystic teratoma (Fig. 34.14), or dermoid cyst, is the most common germ cell neoplasm of the ovary. Lesions contain mature elements derived from the ectoderm, mesoderm, or endoderm, resulting in a broad range of appearance. Mean patient age at discovery is 30 years. Most lesions are discovered incidentally while the patients are asymptomatic. The cysts are filled with liquid sebaceous material that is fat density on MR and CT. Internal contents include the Rokitansky nodule, which commonly includes hair, teeth, bone, or cartilage. US features are usually characteristic, but lesions may be discovered or further characterized by MR or CT. MR shows the sebaceous material as very high intensity on T1WI. Signal is usually decreased on T2WI approximating fat signal. Fat content is confirmed by in-phase and out-of-phase gradient recall images or frequency selective fat-saturation images. CT demonstration of fat density within a cystic adnexal mass is definitive (see Fig. 34.18). CT and plain radiographs show bone and teeth formation within the mass (see Fig. 34.19). Atypical lesions mimic a wide variety of other adnexal pathology including ovarian malignancy (28).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree