▪ Urinary Tract Infections
Urinary tract infection (UTI) is the most common problem of the genitourinary system encountered in children. The urinary tract is the second most common site of infection in children overall, with the upper respiratory tract being the first. The incidence of UTI is higher in girls than in boys, probably because of the short length of the female urethra. There is some current controversy concerning when children with UTIs should be imaged. According to the revised 2011 American Academy of Pediatrics (AAP) guidelines on UTI in febrile infants and young children, voiding cystourethrograms (VCUGs) are no longer routinely performed after the first febrile UTI. The immediate goals of imaging children with UTIs include identifying underlying congenital anomalies that predispose the child to UTI, identifying vesicoureteral reflux (VUR), identifying and documenting any renal cortical damage, providing a baseline renal size for subsequent evaluation of renal growth, and establishing prognostic factors. The long-term goal is to eliminate the chance of renal damage leading to chronic renal disease and hypertension.
The work-up of a child with a first febrile UTI typically involves a renal and bladder ultrasound. However, routine VCUGs after a first febrile UTI are currently no longer recommended. Ultrasound is a poor screening test for the diagnosis of VUR; therefore the current imaging recommendations in young infants and children are widely debated. Under the current guidelines, VCUG should be obtained only if (1) the renal and bladder ultrasound shows hydronephrosis, scarring, obstructive uropathy, or masses; (2) any complex medical condition is associated with the UTI; (3) other findings that suggest high-grade VUR or obstructive uropathy are appreciated; or (4) there is a recurrence of febrile UTI. VCUGs can be performed using fluoroscopy or nuclear medicine. There is some debate about the indications for one or the other of the two studies. The advantage of the fluoroscopic cystourethrogram is that it demonstrates better anatomic detail. The advantage of the nuclear cystogram is that the patient is exposed to less radiation. However, in modern fluoroscopic units with pulse fluoroscopy, the difference in radiation dose between the two techniques is minimal. Because of the need for sharp anatomic detail in all boys and in girls with anatomic abnormalities demonstrated on ultrasound, most would advocate fluoroscopic cystograms for those patients. Nuclear cystograms are adequate for girls with normal ultrasound examinations. In other portions of the globe, the VCUG has been nearly entirely substituted by voiding urosonography, an ultrasound examination using intravesical contrast agent (microbubbles).
Renal Ultrasound
In many pediatric institutions, renal ultrasounds are performed with the patient in both the supine and prone positions. Transverse and longitudinal images are obtained of the kidney and bladder. Renal lengths are measured in both the prone and supine positions but are often more accurate with the patient in the prone position. In every case it is important to compare the patient’s renal length with tables that plot normal renal length against age. In addition, the left and right kidneys should normally be within 1 cm of each other in length. If there is a discrepancy of more than 1 cm, an underlying abnormality should be suspected. A size discrepancy may result from a disorder that causes one of the kidneys to be too small, such as global scarring, or from a process that causes one of the kidneys to be too large, such as acute pyelonephritis or renal duplication.
The kidneys of infants have several characteristics that are different from those of older children and adults. They commonly have a prominent undulating contour. This is a normal appearance secondary to fetal lobulation ( Fig. 6-1 ). In addition, infants’ kidneys can demonstrate prominent hypoechoic renal pyramids (see Fig. 6-1 ) in contrast to the more echogenic renal cortex. These findings should not be mistaken for hydronephrosis.

Magnetic Resonance Urography
With the advent of improved magnetic resonance imaging (MRI) technology, MR urography (MRU) has been increasingly used because of its superb delineation of the anatomy as a result of its intrinsic, high soft tissue contrast resolution and multiplanar three-dimensional reconstruction capabilities. MRU is considered a “one-stop shop” examination because it can provide both anatomical and functional information in one examination without the use of ionizing radiation. MRU plays an important role in the evaluation of ureteral ectopia and genital anomalies. However, the widespread use of MRU is limited by its cost, availability, and the need for sedation.
Fluoroscopic Voiding Cystourethrogram
The most common indication for a fluoroscopic VCUG is the evaluation of UTI. Other indications include voiding dysfunction, enuresis, and the work-up for hydronephrosis. The VCUG demonstrates the presence or absence of VUR and also documents anatomic abnormalities of the bladder and urethra. Because catheterization is used for the procedure, there can be a great deal of anxiety for both the patient and the parents. Education of the parents and patient before the examination is crucial to optimizing the patient’s experience. VCUGs are performed under fluoroscopy with the patient awake. The patient is catheterized under sterile conditions on the fluoroscopy table, typically using an 8F catheter. A precontrast scout view of the abdomen can be obtained to evaluate for calcifications, document the bowel pattern so that it is not later mistaken for VUR, and document the catheter position within the bladder. Contrast is then instilled into the bladder. An early filling view of the bladder should be obtained to exclude a ureterocele. A ureterocele appears as a round, well-defined filling defect on early filling views. On later full views of the bladder a ureterocele can be compressed and obscured. Once the patient’s bladder is full, bilateral oblique views are obtained to visualize the regions of the ureteral vesicular junctions. These views are typically obtained with the collimators open from top to bottom, with the bladder positioned at the inferior aspect of the screen and the expected path of the ureter included on the film. During voiding, the male urethra is optimally imaged with the patient in the oblique projection. The female urethra is best seen on the anteroposterior view. It is critical to obtain an image of the urethra during voiding, particularly in males. In order to ensure that a view of the urethra is obtained, an image should be obtained during urination with the catheter in place and then a second view can be obtained after the catheter has been removed. After the patient has completed voiding, images are obtained of the pelvis and over the kidneys, documenting the presence or absence of VUR and evaluating the extent of postvoid residual contrast within the bladder and renal collecting systems. The use of fluoroscopy should be brief and intermittent during bladder filling. Fluoroscopic last-image hold images can often substitute for true exposures to further decrease radiation dose.
Sometimes, particularly in older children, it may be difficult to get the child to void on the table. Almost all children will eventually void, and a great deal of patience is required during such prolonged examinations. There are several maneuvers that may help the child to void. They include placing warm water on the patient’s perineum or toes; placing a warm washcloth on the patient’s lower abdomen; tilting the table so that the head is up; letting the patient hear the sound of running water in the sink; and dimming the lights.
The expected bladder capacity of small children can be calculated by adding 2 to the patient’s age in years and multiplying that number by 30. This yields the bladder capacity in milliliters. Obviously this formula works only up to a certain age.
Acute Pyelonephritis
There is some confusion concerning the terminology used for infections of the urinary tract in children. The definition of UTI is the presence of bacteria in the urine, but the term typically refers to infections of the lower urinary tract. Acute pyelonephritis is defined as UTI that involves the kidney. Young children may present with nonspecific symptoms, such as fever, irritability, and vague abdominal pain. In older children the findings may be more specific, such as fever associated with flank pain. In patients in whom the diagnosis is straightforward, no imaging is needed during the acute infection. Cortical scintigraphy using dimercaptosuccinic acid (DMSA) has been advocated as being the most sensitive test for the diagnosis of pyelonephritis, demonstrating single or multiple areas of lack of renal uptake of the radiotracer. These areas tend to be triangular and peripheral ( Fig. 6-2 ). Other imaging studies that have also been advocated to detect acute pyelonephritis include color Doppler ultrasound and contrast-enhanced computed tomography (CT). These studies typically demonstrate lack of color flow or contrast enhancement of triangular, peripheral portions of the kidney ( Fig. 6-3 ). CT may show a striated nephrogram ( Fig. 6-4 ). In addition, pyelonephritis can be very focal and can mimic a mass on all of these studies ( Fig. 6-5, A and B ). Ultrasound and CT may also demonstrate disproportionate enlargement and swelling of the affected kidney as compared with the contralateral side. Sometimes the findings of pyelonephritis will be encountered on these imaging studies when the studies were obtained for other suspected causes of abdominal pain, such as appendicitis.




Chronic Pyelonephritis
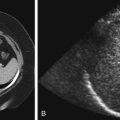
Chronic pyelonephritis is defined as the loss of renal parenchyma resulting from previous bacterial infection. It is synonymous with renal scarring. Normally the renal cortical thickness should be symmetric and equal within the upper, mid, and lower poles of the kidneys. The loss of renal cortical substance as seen by ultrasound, most commonly at one of the renal poles, is suggestive of the diagnosis ( Fig. 6-6 ). This should not be confused with fetal lobulation (also known as an interrenicular septum; Fig. 6-7 ), a normal variant. In pyelonephrotic scarring the indentations of the renal contour tend to overlie the renal calyces, whereas in fetal lobulation the indentations are between renal calyces (see Fig. 6-7 ).


▪ Evaluation of Prenatally Diagnosed Hydronephrosis
As a result of the increasing use of prenatal ultrasound, pediatric radiologists are more frequently having to perform postnatal work-ups of prenatally diagnosed hydronephrosis. The evaluation of such patients typically includes both. The controversy revolves around the timing of the ultrasound evaluation. In neonates there is a relative state of dehydration that occurs after the first 24 hours of life. Reports have shown that this relative state of dehydration can lead to underestimation or nondetection of hydronephrosis by ultrasound. Therefore it is recommended that the postnatal evaluation of prenatally diagnosed hydronephrosis be performed during the first 24 hours of life or after 1 week of age. The disadvantage of doing the ultrasound during the first 24 hours of life is the interruption of mother-child bonding, whereas the disadvantage of performing the examination at 7 days of life is the potential of parental noncompliance and losing the patient for follow-up.
Congenital Anomalies
Congenital anomalies of the genitourinary tract are commonly encountered during work-ups for UTIs or other abnormalities.
Vesicoureteral Reflux
VUR is defined as retrograde flow of urine from the bladder into the ureter. It is thought to be a primary abnormality related to immaturity or maldevelopment of the ureterovesicular junction. Normally the ureter enters the ureterovesicular junction in an oblique manner such that the intramural ureter traverses the bladder wall for an adequate length to create a passive antireflux valve. In VUR the angle of entrance of the ureter is abnormal and the intramural tunnel is short, resulting in VUR. VUR occurs in less than 0.5% of asymptomatic children but is present in as much as 50% of children with UTIs. There is an increased incidence of VUR in siblings of children with VUR and in children of parents who had VUR. VUR incidence is lower in individuals of West African descent. The importance of VUR is its association with renal parenchymal scarring. VUR is present in almost all children with severe renal scarring. In addition, a direct correlation between the grade of VUR and prevalence of scarring has been demonstrated. Other complications associated with VUR include acute pyelonephritis, interference with the normal growth of the kidney, and development of hypertension. The degree of VUR is graded on the basis of several characteristics ( Fig. 6-8 ): the level to which the reflux occurs (ureteral versus ureteral and collecting system); the degree of dilatation; the calyceal blunting; and papillary impressions. Grade 1 reflux is confined to the ureter. Grade 2 reflux fills the ureter and collecting system, but there is no dilatation of the collecting system. Grade 3 reflux is associated with blunting of the calyces. In deciding whether a calyx is dilated or not, it has been taught that if it looks like you could pick your teeth with a calyx, it is not dilated ( Fig. 6-9 ). Grade 4 reflux is identified by progressive, tortuous dilatation of the renal collecting system. Grade 5 reflux is defined by the presence of a very tortuous dilated ureter. Both grade 4 and 5 reflux can be associated with intrarenal reflux. It is surprising, but significant VUR can be present in spite of a normal renal ultrasound. Lack of dilatation on ultrasound in no way excludes the presence of VUR. Dilatation of the ureter or collecting systems can sometimes be seen intermittently when VUR is present.


Most low-grade VUR resolves spontaneously by the age of 5 to 6 years unless there is an underlying anatomic abnormality. In children without anatomic reasons that prohibit spontaneous resolution of reflux, most are treated with prophylactic antibiotics alone, although this is controversial. Antibiotic therapy is discontinued when the reflux has resolved. Surgical reimplantation of the ureter or injection of a bulking agent below the ureteric orifice (minimally invasive endoscopic treatment) is considered when the degree of VUR is severe, if there is evidence of renal scarring, if the VUR has not resolved over a reasonable time, or if breakthrough infections occur frequently. After periureteral injection, ultrasound will show an echogenic mound in the bladder wall in the region of the treated ureteral orifice ( Fig. 6-10 ).

Ureteropelvic Junction Obstruction
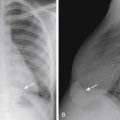
Ureteropelvic junction (UPJ) obstruction is defined as an obstruction of the flow of urine from the renal pelvis into the proximal ureter. It is the most common congenital obstruction of the urinary tract. There is an increased incidence of other congenital anomalies of the urinary tract in patients with UPJ obstruction. They include VUR and renal duplication, among others. Males are more often affected than females, and there is a predilection for the left side. In addition, UPJ obstruction may be present bilaterally, but the severity may be asymmetric. The cause of most UPJ obstructions is intrinsic narrowing at the UPJ. However, extrinsic compression secondary to anomalous vessels is also occasionally identified. On ultrasound there is dilatation of the renal collecting system without dilatation of the ureter ( Fig. 6-11 ). The degree of dilatation may be severe. Renal scintigraphy using technetium 99m mercaptoacetyltriglycine (MAG 3) with diuretic (furosemide) challenge is often used to evaluate the severity of the UPJ obstruction. Most neonates with UPJ obstruction are characteristically asymptomatic, and the condition is usually detected following postnatal ultrasound evaluation of antenatal hydronephrosis. Children with UPJ obstruction and other congenital anomalies are predisposed to renal injury even by minor abdominal trauma ( Fig. 6-12 ). Mild to moderate UPJ obstructions are sometimes treated conservatively, but most urologists treat severe UPJ obstruction surgically, with the gold standard, open pyeloplasty, being increasingly replaced by robotic or laparoscopic pyeloplasty, with similar rates of success.


Multicystic Dysplastic Kidney
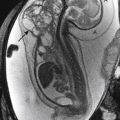
Multicystic dysplastic kidney (MCDK) is thought to be related to severe obstruction of the renal collecting system during fetal development. The site of the obstruction determines the imaging appearance. The most common appearance of MCDK is that of a grapelike collection of variably sized cysts that do not appear to communicate ( Fig. 6-13 ). Historically the diagnosis of MCDK has been made on the basis of DMSA scans demonstrating trace or no renal uptake on 4-hour images. Currently, high-quality ultrasound scans may suffice for the diagnosis in most instances. In patients with MCDK it is important to exclude other associated congenital anomalies of the contralateral kidney. UPJ obstruction is commonly identified. Rarely, MCDK can be isolated to an upper or lower pole.

Most MCDKs slowly decrease in size over time. Often the remaining residual dysplastic renal tissue will no longer be visualized by imaging techniques. Although in the past, nephrectomy was the usual treatment for MCDK, most patients are currently treated nonoperatively. However, they are followed by ultrasound because there is some controversy as to whether there may be an increased risk for developing malignancy in an MCDK. MCDK can also predispose patients to hypertension, and if hypertension develops, nephrectomy is usually performed.
Ureteropelvic Duplications
The term ureteropelvic duplication refers to a broad range of anatomic variations ranging in severity from incomplete to complete. The incomplete form of duplication is more common than the complete form and is the most common anomaly affecting the upper renal collecting system. It occurs most often in females. With incomplete duplication, there can be a bifid renal pelvis, two ureters superiorly that join in midureter, or duplicated ureters that join just before insertion into the bladder wall. With complete duplication there are two completely separate ureters that have separate orifices into the bladder. Ureteropelvic duplication is thought to occur secondary to premature division or duplication of the ureteral bud. Such duplications are five times more common unilaterally than bilaterally. On ultrasound, incomplete renal duplication may appear as an area of echogenicity similar to the renal cortex separating the echogenic central renal fat into superior and inferior components ( Fig. 6-14 ). Noncomplicated, incomplete renal duplications have little significance and should be thought of as a normal variation. Children with incomplete duplication are not at increased risk for urinary tract disease as compared with children without duplications.

In patients with complete ureteropelvic duplication, there is a higher incidence of UTI, obstruction, VUR, and parenchymal scarring. In these patients the ureteral orifice of the upper pole moiety inserts more medially and more inferiorly than the orifice of the lower pole ureter. This is known as the Weigert-Meyer rule. The lower pole system is more prone to VUR. The upper pole system is more prone to obstruction secondary to an ectopic ureterocele ( Figs. 6-15 and 6-16 ).


Ureterocele
A ureterocele is defined as dilatation of the distal ureter. The dilated portion of the ureter lies between the mucosal and muscular layers of the bladder. The ureteral orifice is usually stenotic or obstructed. Ureteroceles are defined as simple when they are positioned at the expected orifice of the ureter at the lateral aspect of the trigone. Ureteroceles are defined as ectopic when they are associated with an ectopic insertion of the ureter. Ectopic ureteroceles can be quite large and are almost always associated with a duplicated collecting system (see Fig. 6-15 ). The ureter from the upper pole moiety is the one associated with the ureterocele. There can be marked associated dilatation of that ureter and the upper pole moiety collecting system. On VCUGs, ureteroceles appear as round, well-defined filling defects, best visualized on early filling views (see Fig. 6-15 ). Ureteroceles may be compressed and not visualized when the bladder is distended by contrast. Sometimes the ureteroceles can invert and appear as diverticula. On ultrasound, typically a dilatated ureter is seen to terminate in a round, anechoic intravesicular cystic structure (see Fig. 6-15 ). Usually there is associated dilatation of the upper pole moiety.
Renal Ectopia and Fusion
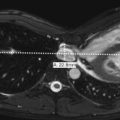
Renal ectopia is defined as abnormal position of the kidney. It results from abnormal migration of the kidney from its fetal position within the pelvis to its expected position in the renal fossa. Renal fusion is defined as a connection between the two kidneys. It results from failure of separation of the primitive nephrogenic cell masses into two separate left and right blastemas. With ectopia the ectopic kidney most commonly lies within the pelvis. Most ectopic kidneys are also malrotated. With crossed fused renal ectopia, both kidneys lie on the same side of the abdomen and are fused ( Fig. 6-17 ). In this anomaly, both ureters drain normally in their expected positions. Single kidneys and crossed fused ectopia are commonly observed in association with VACTERL (vertebral defects, anal atresia, cardiac defects, tracheo-esophageal fistula, renal and limb abnormalities). The most common type of renal fusion is horseshoe kidney, which occurs in approximately 1 in 400 live births. With horseshoe kidney there is fusion of the lower pole of the two kidneys across the midline. The connecting isthmus may consist of functional renal tissue or fibrous tissue and cross the midline anterior to the aorta and inferior vena cava. Most horseshoe kidneys are located more inferiorly than normal. Horseshoe kidneys may be seen in association with Turner syndrome (XO) and trisomy 18. The number of ureters arising from a horseshoe kidney is variable, and they exit the kidney ventrally rather than ventromedially. Horseshoe kidneys and other types of renal fusions are at increased risk for infection, injury from mild traumatic events ( Fig. 6-18 ), renal vascular hypertension, stone formation, and hydronephrosis ( Fig. 6-19 ). There is also a slight increase in incidence of Wilms tumor. On ultrasound, horseshoe kidney may be more difficult to diagnose on the standard longitudinal and axial planes than one might expect. It may also be difficult to obtain accurate measurements of the kidneys in the longitudinal plane because of the poorly defined inferior pole. If the ultrasound probe is moved posterolaterally and the patient is scanned in the coronal plane, the two kidneys and connecting isthmus can be visualized on a single image ( Fig. 6-20, A-C ). Such images are most easily obtained in infants. Horseshoe kidneys are readily visualized on CT scanning (see Fig. 6-20 ).