4
Genitourinary Brachytherapy
André-Guy Martin, Frédéric Lacroix, Thomas Niedermayr, Paul L. Nguyen, and Peter F. Orio, III
The predominant form of genitourinary brachytherapy performed worldwide is prostate brachytherapy, either low dose rate (LDR) or high dose rate (HDR). Rarer tumors such as penile cancer can also be treated with brachytherapy, often as the preferred approach due to its organ-sparing capability. Female urethral cancer is often treated with brachytherapy by the gynecologic radiation oncologists due to the similarity in technique to a vaginal template implant. Brachytherapy for male urethral cancer often utilizes an implant approach as for that of penile cancer. Brachytherapy for bladder cancer is rare but has been described with successful organ-conserving results (1), even utilizing modern laparoscopic catheter placement techniques (2). Thus, this chapter describes in depth prostate brachytherapy and also presents some case vignettes that introduce the role of penile brachytherapy.
In the United States, prostate cancer is the most common (excluding nonmelanoma skin tumors) malignancy and the second cause of cancer-specific deaths in men (3). With the advent of prostate-specific antigen (PSA) screening in the late 1980s, most men being diagnosed with prostate cancer today have early-stage disease (3,4). Treatment options for these men can include radical prostatectomy (RP), brachytherapy, external beam radiotherapy (EBRT), or active surveillance.
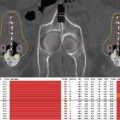
For brachytherapy, there are now 10- to 15-years of published data that support the excellent treatment outcomes that are at least comparable to those of RP or EBRT (Table 4.1) (5−9). Some of its advantages can include a more rapid postoperative recovery time when compared to RP and a shortened overall treatment time when compared to EBRT. Brachytherapy also has the advantage of being a much less expensive form of radiation with a cost to Medicare of $17,076 per patient as compared to $31,574 for intensity modulated radiation therapy (IMRT) (10).
Relatively recent important advances in imaging and procedural technologies have also enabled prostate brachytherapy to evolve further into a minimally invasive, state-of-the-art, computerized planning software-aided treatment approach. Since its inception as a freehand open laparotomy procedure in the 1960s at Memorial Sloan-Kettering Cancer Center (MSKCC), permanent interstitial prostate brachytherapy has benefited from the development of transrectal ultrasonography guidance approach, template-guided closed transperineal technique, and sophisticated commercialized planning software technology for CT-based and MRI-assisted postoperative dosimetry.
Table 4.1 Data supporting the treatment outcomes

As these technological advances become more refined, the prostate brachytherapy procedure continues to adapt and evolve, with the goal of maximizing the therapeutic ratio. This chapter reviews the basic modern technique as well as innovations in imaging, seed loading, and real-time planning for LDR and HDR afterloading brachytherapy.
PATIENT SELECTION FOR PROSTATE BRACHYTHERAPY
Low-risk and favorable intermediate-risk disease: Consistent with both the National Comprehensive Cancer Network (NCCN) guidelines and the American Brachytherapy Society (ABS) guidelines, prostate brachytherapy monotherapy has traditionally been offered for patients with low-risk (cT1c–T2a, PSA < 10, and Gleason 6) disease. However, both guidelines also endorse brachytherapy for select patients with intermediate-risk disease and there is evidence of excellent results, especially for patients with “favorable” intermediate-risk disease, which has been variably defined, but might include patients who have Gleason 7 or PSA 10 to 20 but not both, or, alternatively, Gleason 3 + 4 = 7 but not 4 + 3 = 7 (usually with less than 50% of the cores positive). Cosset et al published their experience with iodine-125 (125I) monotherapy (with a D90 of typically 180 Gy) and found that the 5-year PSA failure-free survival for patients with favorable intermediate risk was 95% (Gleason 6 with PSA 10–15) and 94% (Gleason 7 with PSA < 10), which was similar to the 97% 5-year PSA control for low-risk patients. However, those who had both a PSA of 10 to 15 and Gleason 7 had only an 88% control, suggesting that additional therapy may be needed for these less favorable intermediate-risk patients (11). For patients with intermediate-risk disease in whom brachytherapy monotherapy is being considered, endorectal MRI may also be a useful test to rule out occult extracapsular extension (ECE). A recent study of 3 Tesla MRI in men with favorable-risk disease found a 75% and 95% accuracy of prediction for ECE and seminal vesicle invasion (SVI), respectively, at the time of prostatectomy (12).
Absolute and relative contraindications: Current ABS guidelines list as absolute contraindications limited life expectancy, unacceptable operative risks, distant metastases, absence of rectum precluding trans rectal-ultrasound (TRUS) guidance, large trans-urethral resection of the prostate (TRUS) defects precluding good dosimetry, and ataxia-telangectasia (13).
The ABS relative contraindications include factors that might lead to a more difficult implant or difficult postoperative course, but which may not preclude brachytherapy by an experienced team. A high International Prostate Symptom Score (IPSS; usually greater than 15–20) has been associated with a higher risk of postoperative retention (14,15). For example, one study suggested that the rate of urinary retention for patients with IPSS > 20 was 29% versus 11% for IPSS 10 to 19 and 2% for IPSS < 10 (15). However, prolonged catheterization beyond 1 week to relieve urinary retention is rare, and prophylactic use of α-blockers can reduce the time course of urinary obstructive symptoms (16). Patients with prior pelvic radiotherapy should generally avoid further radiation, but often these patients are also not candidates for surgery due to the risk of rectal injury. In the salvage brachytherapy literature, the risk of rectal fistula nationwide was 3.4% (17). Any attempt at brachytherapy in patients who have been previously irradiated should proceed very cautiously (18).
Prior, TURP is a relative contraindication because good dosimetry can be more difficult to achieve, but a series with 171 patients from the Chicago Prostate Center (19) and a smaller series from MSKCC (20) have indicated good outcomes after TURP. Large glands greater than 60 cc can prove challenging due to increased pubic arch interference and potentially an increased risk of urinary retention. However, the pubic arch interference can often be overcome with extended lithotomy, and another common practice is to give 2 to 3 months of neoadjuvant androgen deprivation therapy (ADT), although there have been recent studies suggesting that ADT to shrink the gland prior to brachytherapy in a typically low-risk population could be used with caution in older men with prior cardiovascular comorbidities (21,22). Similarly, patients with enlarged median lobes protruding into the bladder neck may be at a higher risk of urinary retention. One strategy might be to avoid implanting the median lobe, particularly in men with low-risk disease in whom the risk of disease in the median lobe is low. One series of eight men in whom the median lobe was implanted reported that two of eight went into urinary retention, and one required intermittent self-catheterization for 3 months (23).
The last of the ABS relative contraindications is inflammatory bowel disease (IBD). A prior series from Massachusetts General Hospital patients with IBD treated with external beam from 1970 to 1999 noted a 46% rate of severe toxicity with 21% needing to stop treatment (24), but symptoms may not be as severe with brachytherapy where the amount of rectum exposed to radiation is much smaller. Other series have suggested that for patients with controlled IBD, there is no increased risk of gastrointestinal toxicity after brachytherapy (25,26).
Special Populations
Young patients: Increasingly, younger patients are becoming interested in brachytherapy as a means of treating prostate cancer with less risk of erectile dysfunction than surgery (27,28). As the follow-up times in brachytherapy series have lengthened, long-term results appear to be excellent. Studies that have evaluated the impact of age on brachytherapy outcomes have consistently found that younger patients (variably defined as younger than 60 years, younger than 54 years, and younger than 50 years) have outcomes that are equivalent to older patients (29−32). In a series of 42 patients aged 50 years or younger who received brachytherapy, recurrence-free survival was 97.7% and potency preservation was 75.6% after 5.6 years of follow-up (29).
Obese patients: Obesity is known to be an adverse prognostic factor after both RP (possibly due to greater difficulty visualizing the operative field [33,34]), and after EBRT (possibly due to greater organ motion leading to geographic misses, or daily changes in position of an abdominal pannus changing the shape of the dose distribution [35,36]). However, after brachytherapy, obesity does not appear to be prognostic, perhaps because direct implantation of the seeds into the prostate under ultrasound guidance eliminates the problem of geographic miss (37,38). Therefore, brachytherapy may be the best choice of therapies for obese patients when choosing among surgery, EBRT, and brachytherapy.
Brachytherapy Boost in Intermediate- and High-Risk Disease
For intermediate-risk disease, both NCCN and ABS guidelines suggest consideration of using either LDR or HDR brachytherapy as a boost along with 45 to 50.4 Gy of EBRT. For patients with high-risk disease, brachytherapy should generally be used only as a boost in combination with EBRT plus androgen deprivation therapy. Hoskin has published a trial of mostly intermediate- and high-risk patients, in which men were randomized to either 55 Gy in 20 fractions, or 35.75 Gy in 13 fractions followed by an 8.5 Gy × 2 HDR boost. Biochemical recurrence-free survival was significantly better (66% for brachytherapy boost vs 48%) at 7 years for brachytherapy boost versus no boost (log rank P = .04), although there has not yet been any difference in overall survival (39). Most recently, the ASCENDE-RT Canadian randomized trial involving 276 high-risk and 122 intermediate-risk men was presented at the 2015 Genitourinary Cancers Symposium in Orlando, Florida. All men received 1 year of ADT and pelvic radiation to 46 Gy in 23 fractions and randomization was to completion of prostate EBRT to 78 Gy in 2 Gy fractions or 125I LDR boost with 115 Gy. Biochemical recurrence-free survival at 9 years was 83% for LDR boost versus 63% for EBRT alone (P = .0022), although no difference in metastases or cancer-specific mortality was seen. There was a higher rate of late urethral strictures in the LDR boost arm (8% vs 2% [40]).
TOXICITY
Urinary
Urinary retention is a multifactorial problem. Before brachytherapy, many patients have urinary dysfunction secondary to benign prostatic hypertrophy (BPH), preexisting prostatism, and small-vessel disease. In a review by Stone et al, the rate of acute urinary retention (AUR) following prostate brachytherapy was found to be between 1.5% and 22% (41). The IPSS pretreatment prostatic volume, urinary flow studies, and the number of needles inserted have all been found to be associated with AUR (42−44).
Detailed patient-reported quality-of-life studies suggest that, by 1 year after the implant, most patients’ urinary symptoms have returned to levels close to their baseline prebrachytherapy state (45,46). For patients who have persistent long-term urinary symptoms after brachytherapy, TURP has been associated with a high rate of urinary incontinence (12/44 = 27% and 7/38 = 18%) and therefore should only be undertaken after a very careful consideration of the risks and benefits (47,48).
Rectal
In the acute setting, symptoms can include urgency, diarrhea, proctitis, and/or irritation of hemorrhoids. The onset of symptoms varies depending on the isotope used in the treatment (49). One large prospective assessment found that the rectal quality of life returns close to the baseline about a year after implantation. A survey of patients conducted by Merrick et al found less than 20% of patients to have worse bowel function following the implant but there were no severe changes in late bowel function (50). The rectal V25 has been associated with worse late diarrhea and V10 > 40% of prescribed dose has been implicated in long-term toxicity (51).
Impotence
The incidence of impotence in long-term follow-up of prostate brachytherapy ranges from 15% to 51% (52). In one large prospective study of 1,200 men published in JAMA, among men who had adequate sexual function pretreatment, the proportion with sexual dysfunction reported at 2 years after brachytherapy was 37%, which was lower than the 60% who reported sexual dysfunction 2 years after surgery (27). The Canadian SPIRIT trial randomized 34 men to either surgery or brachytherapy and prospectively followed 154 men who chose their treatment. In a combined analysis of all patients in the study, brachytherapy was associated with significantly better sexual domain scores 5 years after treatment (52.5% vs 39.2%; P = .001 [28]). Factors that have been related to impotency include pretreatment potency, microvascular damage, radiation dose to the penile bulb and neurovascular structures, diabetes, and age. Merrick et al have studied the dose to the neurovascular structures and penile bulb (53,54). They found no difference in the dose to the neurovascular structures in patients who were impotent compared with those who were potent. The dose to the penile bulb, however, along with pretreatment potency, was related to erectile dysfunction on multivariate analysis (54).
RADIATION SAFETY
For the families of patients with permanent radioactive seed implants, many questions are raised regarding radiation safety. Studies looking at the measured radiation dose rate at the patient’s surface and at 1 m from the patient’s surface have determined that these patients do not represent a risk to the general public or to their families (55,56). One study attached radiation dosimeters to spouses of patients receiving brachytherapy and found the total lifetime exposure of the spouse due to the implant was a mean of 0.10 mSv for 125I and 0.02 mSv for palladium-103 (103Pd) implants, which is less than the 0.12 mSv of total exposure received when flying round-trip from New York to San Francisco (57). To follow general as low as reasonably achievable (ALARA) principles, patients should, however, be given written documentation regarding radiation safety and precautions that can be taken when they are in the presence of small children and pregnant women.
Medical Events in Prostate Brachytherapy
In the United States, a medical event has been defined by the Nuclear Regulatory Commission (NRC) under the Code of Federal Regulations (CFR) 10 Part 35 as when “the total dose delivered differs from the prescribed dose by 20 percent or more.” However, this definition was problematic for prostate brachytherapy. For example, an excellent implant that met all standard metrics intraoperatively could be deemed a medical event if temporary postoperative swelling of the prostate caused the Day 1 dosimetry to appear 20% cooler than the planned dose. The calculated dose to the prostate could vary considerably based on the amount of swelling of the prostate and the timing of the postoperative dosimetry. Therefore, in 2011, ASTRO convened a working group, which recommended to the NRC that the definition of medical events for prostate brachytherapy should not be based on dose (58). Rather, they recommended that a medical event be defined as a situation in which greater than 20% of the source strength prescribed in the postprocedure written directive was deposited outside the planning target volume. This definition is not affected by prostate swelling and was felt less likely to generate spurious medical events than the original dose-based definition. On July 9, 2013, the NRC released a notice that it was working on revising these regulations and was considering eliminating dose-based definitions of medical events for prostate brachytherapy. In the interim, it would allow for flexibility in enforcements of violations that would have been acceptable under a source-strength definition of medical events (59).
ULTRASOUND-GUIDED PERMANENT SEED IMPLANTATION OF THE PROSTATE
As discussed previously, LDR prostate brachytherapy (8,60−62) may be used as monotherapy for lower risk prostate cancer patients as well as in conjunction with EBRT in the treatment of higher risk disease (63−65). Treatment-planning principles, implantation techniques, and side effects associated with LDR prostate brachytherapy are reviewed in the following.
Assessment of Prostate Volume and Geometry
If a patient meets appropriate criteria for an LDR prostate implant, the size of the prostate and the geometry of the prostate in relationship to the pubic arch will need to be determined. In our practice of intraoperatively planned implants, prostate volume studies are accomplished in one of two ways, either with a TRUS or by performing a CT scan of the prostate and pubic arch in a modified dorsal lithotomy position. Both are accurate in determining the number of seeds needed for intraoperatively planned implants as well as for determining the potential for pubic arch interference at the time of implant. Nomograms exist to estimate the number of seeds needed for an implant based on isotope activity and prostate volume. Centers are encouraged to construct their own nomograms with information from their actual implant techniques to determine the activity and number of seeds needed for their particular implant style (Figure 4.1) (66).
When preplanning techniques are utilized for the creation of the seed and needle positions prior to the day of the implant, a TRUS volume study is performed and serves multiple purposes. In addition to determining the size and shape of the prostate gland, the geometry of the prostate to the pubic arch is also assessed (67). The ultrasound images are then imported into the treatment planning system to generate a plan with appropriate needle and isotope placement within the prostate. With this approach careful measurements are recorded to help ensure that the patient will have a reproducible position at the time of implant. When intraoperative planning techniques are utilized for implant, consideration to the treatment planning system is necessary. Commercially available systems can differ between how images are acquired for volume study, either transverse or sagittal. On most ultrasound probes the sagittal crystal is proximal to the transverse crystal, which has implications of the needed depth of insertion to visualize the prostate, seminal vesicles, and bladder.

Figure 4.1 Nomogram of implanted activity (mCi) versus implant volume (cc) based on departmental data for 125I.
Volume Study
Both preplanned and intraoperative planning will require an assessment of the prostate in relationship to the pubic arch and to organs at risk (OAR) including the urethra, bladder, and rectum. When a TRUS volume study is performed outside of the operating room (OR), the patient is asked to have a comfortably full bladder and is placed in the dorsal lithotomy (treatment) position. Depending on the commercial treatment system being utilized, images are acquired either transversely or from a sagittal reconstruction. Described first is a technique utilized by transverse-based planning systems. The rectal ultrasound mount is secured to the examination table or the floor and the rectal ultrasound transducer is secured onto the stepper (Figure 4.2). The angle of the mount and transducer is recorded. A cover is secured over the transducer and the apparatus is then lubricated with ultrasound jelly and gently inserted into the rectum. The best visualization of the prostate comes when there is no air interface between the ultrasound transducer and the rectal wall. Once the ultrasound transducer is in position, the bladder, prostate, seminal vesicles, and rectum can be identified. The technique developed at the Seattle Prostate Institute (67) establishes the base of the prostate first, which can be found at the proximal end of the prostate interface with the bladder. This interface can be best confirmed on a sagittal view (Figure 4.3). Once the base of the prostate is identified, the prostate is contoured in 5 mm increments from the base to the apex. The physician can then define a margin around the prostate as a target to be implanted, typically 3 to 5 mm in all directions except posterior where no margin is planned. Once the prostate gland has been defined, the number of contours should be compared with the length of the prostate measured on the sagittal views to ensure consistency. The volume of the gland should also be recorded. To determine pubic arch interference, a tracing of the pubic arch (Figure 4.4) is superimposed over each defined slice of target volume. If there is no pubic arch interference and the prostate volume is within the defined limits, the contours and images can be transferred to a treatment-planning system for prostate brachytherapy. If the prostate gland is large or there is arch interference secondary to gland size, neo-adjuvant androgen suppression can be used to shrink the gland, with a rule of thumb of 3 to 4 months of a luteinizing hormone releasing hormone (LHRH) agonist resulting in appropriately a one-third reduction in prostate size (68,69). However, the risk of urinary symptoms that are associated with a large gland do not necessarily decrease when the gland size is decreased with androgens (70). If the clinician is concerned about the subjective urinary symptoms reported by a patient, then formalized urinary dynamics and post void residual volumes should be considered.

Figure 4.2 Typical transrectal ultrasound (TRUS) volume study setup.
Figure 4.3 Sagittal view of the prostate on transrectal ultrasound. The bladder prostate interface is most easily distinguished on sagittal imaging.

Figure 4.4 Axial ultrasound image of prostate with pubic arch defined.
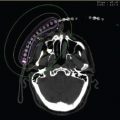
In our current practice of interactive intraoperative planning utilizing sagittal image guidance of needle positioning and placement, which is described in detail subsequently, volume studies are performed with either TRUS or with CT scan performed in the modified lithotomy position. When utilizing a TRUS sagittal volume study the technique is similar to transverse volume study as described earlier but the process is automated. Instead of stepping the probe out of the rectum from base to apex as described previously, the probe is inserted into the rectum allowing sagittal viewing of the prostate, the entire seminal vesicle, and the proximal prostate–bladder interface. The probe is then automatically rotated in a 140º sweep scanning the entire prostate. The prostate is reconstructed in the transverse, coronal, and sagittal planes for treatment planning. A CT-based technique can also be used to delineate the prostate volume in a modified dorsal lithotomy position (Figure 4.5), which also allows for three-dimensional (3D) contouring of the prostate and pubic arch. A 3D reconstruction of the prostate and the pubic arch is easily accomplished in most external beam treatment planning systems in commercial use today. Geometric assessment of the prostate to potential pubic arch interference is performed (Figure 4.6A without pubic arch interference and Figure 4.6B with pubic arch interference). When CT scans are used for our volume studies, a TRUS-based volume study is performed intraoperatively for treatment planning.
Figure 4.5 CT scan with patient in modified dorsal lithotomy position for geometric assessment of prostate to the pubic arch.


Figure 4.6 (A) Geometric assessment of the prostate to pubic arch using CT scan and 3D rendering with external beam planning system (eclipse). Images demonstrate no prostate–pubic arch interference. (B) Geometric assessment of the prostate to pubic arch using CT scan and 3D rendering with external beam planning system (eclipse). Images demonstrate prostate–pubic arch interference. 3D, three dimensional.
Seed Selection
Isotope
125I and 103Pd are the most commonly used isotopes in LDR brachytherapy for prostate cancer and both have demonstrated excellent long-term clinical outcomes in terms of efficacy and safety. Cesium-131 (131Cs), which was introduced in 2004, is a more recent option for LDR implant. Historically, 125I was the first isotope used with modern TRUS-guided implants, although all isotopes are capable of providing excellent dosimetry. Each isotope requires a thorough understanding of the characteristics of the isotope and how planning and final dosimetry may be influenced by implant style. Currently 125I is the most frequently implanted isotope. Randomized trials have not found differences in biochemical-free survival between 125I and 103Pd (71). There are several centers that use 103Pd and 131Cs effectively and some brachytherapists use different isotopes based on clinical situations. It has been suggested that 103Pd is more effective against dedifferentiated tumors than 125I (72). Through extrapolation, a similar assertion could be made for 131Cs; however, no differences in clinical outcomes for patients with prostate cancer treated with either 103Pd or 125I have been found (73,74). The ABS (13) does not recommend one specific isotope over another, although the longest toxicity data exist for both 125I and 103Pd. The dosimetric properties of these three isotopes are compared in Table 4.2.
Table 4.2 125I, 103Pd, and 131Cs properties

Loose Versus Stranded Seeds
Isotopes are available as either loose seeds or stranded seeds (Figure 4.7). Commercial systems exist to link seeds together using real time data created from intraoperative planning, as we currently use in our practice (Figure 4.8). Loose seeds are associated with a greater likelihood of seed migration (75,76). However, with very few exceptions seed migration in most cases is not clinically worrisome (77−80). The incidence of seed migration is decreased with stranded seeds as compared with that of loose seeds (risk ratio [RR]: 3.08 vs 6.97), with the greatest difference being in migration to the lung and perineum (81). Dosimetrically, the two appear to be very similar. It is also possible to perform implants simultaneously utilizing both loose and stranded seeds with excellent dosimetry and clinical outcomes. Interpretation of the literature requires care, as implant technique can influence dosimetry as much as implanting loose or stranded seeds. A prospective trial demonstrated that although 15% of strands can shift 5 mm or more within the gland over 4 weeks, the impact on dosimetry was negligible (82). Knowledge of the anatomy of the prostate and surrounding tissues helps to minimize seed migration. Migration pathways of seeds are typically associated with the deep dorsal vein and neurovascular bundle. The venous drainage of the prostate starts with the deep dorsal vein. The vein has three major branches, the superficial branch and the right and left branch. The superficial branch lies on top of the prostate and bladder neck and drains into the dorsal venous complex. Care should be taken to delineate the prostate from the venous structures so as not to place seeds in this area that have the potential to migrate. Furthermore, the neurovascular bundle typically lies at 5 and 7 o’clock positions and is another potential pathway for seed migration. Duplex ultrasound allows the clinician to identify blood flow and thus allow the identification of these structures when not grossly apparent.
Activity
The average activity of 125I seed used in prostate cancer is 0.41 mCi (range: 0.16–1 mCi) and 1.32 mCi (range: 0.50–1.90 mCi) for 103Pd (83). Historically, the Seattle group used low-activity 125I seeds (0.35 mCi) to improve dose homogeneity within the prostate as well as decrease the dose to the rectum and urethra (84,85). A dose study performed by D’Souza et al compared dose homogeneity among different seed strengths (0.35, 0.44, and 0.66 mCi). They found the 0.44 mCi-activity seed to have the best dose distribution (86). Narayana et al (87) performed a randomized trial between high-activity 125I seeds (0.60 mci) and low-activity 125I seeds (0.31 mCi) and found excellent dosimetry with both high and low activities. The implant technique is likely just as important as the activity of seed implanted. With the use of higher activity seeds, fewer seeds are required to cover the target volume, especially in peripheral loading techniques. The placement of each seed becomes more important where deviations in planned placement from actual placement have the potential to increase toxicity to normal structures or to under dose the target (88). This has become apparent in our clinical practice. When using intraoperative planning, it is possible to devise plans with intended coverage dependent on one or two well-placed seeds, which is magnified with increasing isotope activity. The overall number of seeds has a direct impact on the number of needles used during the procedure. Eapen et al (42) have reported that needle trauma to the prostate contributes to acute urinary toxicity. One could argue that the placement of more needles well on the first pass may in theory be less traumatic than placing fewer needles multiple times. Therefore, we try to take great care to ensure that the needle is on the correct trajectory before puncturing the prostate.

Figure 4.7 125I loose seeds and stranded seeds (QUICKLINK®).

Figure 4.8 Bard QUICKLINK® used intraoperatively to link seeds based on real-time planning.
Treatment Planning
Prescription Dose
The ABS recommends prescription doses of 145 Gy and 125 Gy for 125I and 103Pd, respectively, in monotherapeutic brachytherapy. In the setting of utilizing seeds as a boost in conjunction with 41.4 to 50.4 Gy of EBRT, the recommended prescription doses are 108 to 110 Gy with 125I and 90 to 100 Gy with 103Pd (13). Typically, these doses are prescribed to the minimum peripheral dose (MPD; Figure 4.9). The MPD is considered to be the maximum dose that covers 100% of the target volume. This dose is dependent on the position of the seeds within the prostate and the dose may vary by up to 25%. Despite this, usually 90% of the target volume will receive the prescription dose (89). The mean peripheral dose is the average of the dose at the surface of the target volume and varies less with seed position. Some centers will therefore prescribe the mean peripheral dose.
Figure 4.9 Minimum peripheral dose schematic showing D90. Courtesy Anthony L. Zietman, MD.

Figure 4.10 Uniform loading of an 125I implant.
Loading Techniques
Many variants of loading techniques exist in treatment planning for transperineal implantation of the prostate. Over the years, techniques have evolved to provide the prostate with curative doses of radiation while decreasing the radiation dose to surrounding normal tissues of the urethra and rectum. One of the earliest techniques, uniform loading, utilized low-energy seeds placed at fixed distances from each other throughout the entire prostate (Figure 4.10). This technique gave a high dose of radiation to the length of the prostatic urethra, as the urethra was not intentionally spared. To spare the urethra while maintaining coverage to the target, degrees of peripheral loading were developed including modified uniform loading, nonuniform loading, and peripheral loading. These techniques shared the principle of decreasing the number of seeds centrally, which had the greatest impact on urethral doses. In modified uniform loading, two thirds of the planned seeds occupy the posterior border of the prostate and the base. Nonuniform loading avoids loading seeds in the central aspect of needles inserted close to the urethra, and peripheral loading (Figure 4.11) uses higher-activity seeds with the posterior implant plane 5 mm anterior to the posterior border of the prostate (90). Attention to the OAR, as well as dose to the prostate, have become more important as greater attention is focused on decreasing the toxicities of implants. Breakthroughs in computing power have brought inverse planning algorithms to the OR where doses to the OAR and prostate are inversely planned with commercially available treatment planning systems. As such techniques exist to carve out the V125 isodose line to the urethra with asymmetrical loading patterns and inverse planning (Figure 4.12).

Figure 4.11 Peripheral loading of an 125I implant.

Figure 4.12 Inversely planned intraoperative 125I implant. Notice V125 isodose line sparring the urethra.
Our intraoperative inversely planned technique are described in detail as follows. This approach allows for a greater freedom of needle and isotope placement within the gland during an intraoperative implant. The brachytherapist is able to modify the dose constraints to the target and OAR, which build on principles of peripheral implants, but with greater abilities for dose shaping of the target volume and collateral tissues. Also strict limits to where seeds can be placed and doses to the OAR can be easily incorporated. In this way, the freedom of inverse planning can be utilized while still maintaining rules previously discussed with peripheral loading techniques.
Procedure
High-quality LDR prostate implants can be accomplished with both preplanning and intraoperative techniques. Intraoperative planning and isotope delivery can be accomplished in several ways. The simplest definition of intraoperative treatment planning is that, within the OR, the patient and probe are not moved between volume study and seed insertion. This can be accomplished in three general ways (91). The first is intraoperative preplanning, which is the creation of a plan in the OR just before the implant procedure, with immediate execution of the plan without movement of the probe and the patient. The second general way is interactive intraoperative planning, which allows for the stepwise refinement of the treatment plan using computerized dose calculations derived from image-based needle position feedback. The third way is dynamic dose calculations, which is the constant updating of dose distribution using continuous deposited seed position feedback. This last way is the subject of intense interest and research as this would allow for real-time postimplant dosimetry, but technical limitations of seed identification currently limit the utility of this approach. Techniques utilized for both preplanning as well as two versions of interactive intraoperative techniques utilized at our institution are presented as follows.
PREPLANNING TECHNIQUE
Under spinal or general anesthesia, the patient is positioned in the lithotomy position utilized at the time of the initial volume study to ensure the same couch angle and ultrasound probe angle. Care is needed to accurately duplicate the position of the prostate for which the treatment plan was designed. A Foley catheter or a gel–air mixture–filled catheter is then placed within the urethra. With newer generation ultrasounds, a Foley catheter may prove sufficient to identify the urethra without the need for a gel–air mixture. The applicator template is secured to the TRUS apparatus. Figure 4.13 depicts the classical manually loaded technique. The image plane is confirmed by scaling through the length of the prostate with the ultrasound stepper to ensure that the images correspond to those taken at the time of the volume study. The reference plane 0.0 (base of the prostate) is located (67). The base location becomes the zero plane on the ultrasound stepper. The physician is guided by a preprinted legend of the needle location, plane, and number of seeds per needle (92). Preloaded needles are kept within a shielded vault (Figure 4.14) until the physician is ready to insert the individual needle. Each hollow bore needle has a sharp beveled edge and a central stylet (Figure 4.15). When the needles are preloaded, the seeds sit within the hollow needle with a bone wax plug at the tip preventing them from dislodging. The central stylet is posterior to the seeds and can be secured in position with a gasket. When removing the needles from the vault, it is important to hold the needle horizontally with the bevel up to avoid dislodging the seeds. Following the preplan legend, the needle is inserted into the correct template location and gentle pressure will advance the needle through the perineal skin and into the prostate. The ultrasound images in both the axial and sagittal views guide the placement of the needle. Rotation of the needle, if it is in the correct plane of view, shows the beveled edge, sometimes known as the hamburger sign (Figure 4.16). To “drop” the seeds within the prostate, the central stylet is held securely in place and the outer needle is slowly pulled back along the stylet. If the stylet is advanced into the needle, it will push the seeds out of the needle and clump them within the prostate. As such it is important to “sow the seeds” as the quality of the implant will be dependent on depositing the seeds as the needle is retracted rather than pushing the seeds into the prostate. Holding the stylet firmly without motion and retracting the needle to the stylet will result in the proper deposition of the seeds within the prostate. Figure 4.17 shows an example of the intended linear alignment and spacing of seeds.
Figure 4.13 Typical ultrasound preplan schematic with prostate and organs contoured. Planned isodose distribution is shown. Used with permission of the Seattle Prostate Institute.

Figure 4.14 This shielded aluminum needle holder is used in the operating room to hold numbered needles between loading and delivery of seeds. The grid numbering is the same as the perineal template. The unit may be sterilized rapidly.
Figure 4.15 Prostate implant needle with central stylet.

Figure 4.16 Axial view of ultrasound showing the beveled edge of a prostate needle. Used with permission of the Seattle Prostate Institute.
Some brachytherapists use stabilizing needles to minimize prostate motion during the implant (Figure 4.18) (93,94). Whether stabilizing needles are used or not, careful attention to the location of the base of the prostate is necessary throughout the entire implant to ensure that the isotopes are deposited as they were planned. Several techniques can be found in the literature. Of interest is the Seattle technique of a two-stage needle system to minimize prostate movement, improve needle loading, and decrease surgical time. This technique involves the insertion of sleeves to the base of the prostate, allowing needles with spacers equivalent to the retraction plane at the tip end to be inserted after confirmation of sleeve placement has been made (95). Several preplanning techniques exist and the right one is the one the brachytherpaist feels comfortable with and has spent time learning to execute well.
A plain film is routinely taken on completion of the implant to evaluate symmetry and compare seed placement to the preplan. Seeds that have migrated to the perineum will also be visible. Bladder irrigation and cystoscopy can be performed to evacuate migrated seeds to the bladder as well as eliminate potential blood clots in the bladder. Cystoscopy has essentially been eliminated in our intraoperative technique, as high-quality ultrasound in combination with sagittal image guided seed placement, makes violation of the bladder unlikely.
Figure 4.17 Anteroposterior (AP) X-ray of a male pelvis showing a prostate implant with linear deposition of radioactive seeds. Used with permission of the Seattle Prostate Institute.

Figure 4.18 Stabilizing needles inserted to the base of the prostate to help limit prostate motion during implant.
Following the procedure, the urethral catheter is removed and the patient remains in the recovery area until he is able to urinate. Patients are counseled on seed migration and radiation safety. Recommendations regarding condom use during intercourse and potentially finding seeds after urination are reviewed. Discharge medications include an α-blocker, an anti-inflammatory, urinary anesthetics, and a short-course antibiotic.
INTRAOPERATIVE TECHNIQUE
Image Acquisition
In the OR, an ultrasound system with a biplanar axial/sagittal transducer is used to acquire the ultrasound images of the prostate, seminal vesicle, dorsal venous plexus, urethra, rectum, bladder, bladder neck, neurovascular bundle, and external urinary sphincter. A Foley catheter is placed to facilitate localizing the urethra as it courses through the prostate. The probe is mounted to a stepper (Figure 4.19), which is rotated along its axis for longitudinal image acquisition through the Oncentra Prostate™ (Nucletron, an Elekta company [Elekta AB, Stockholm, Sweden]) treatment planning software-controlled motor. During image acquisition, the software captures live images every 0.5º as the motor rotates the probes along a 140º sweep of the prostate and OAR. These sagittally acquired images are the input for a 3D image reconstruction, which provides axial, sagittal, and coronal planes of the acquired volume (Figure 4.20). An 18G template is mounted on the stepper for needle insertion, previously calibrated to the digital template in the planning software for geometrical integrity. Axial images are reconstructed from the 140º sweep at 1.0 mm increments. At a minimum, the prostate, urethra, and rectum are contoured on these axial images while checking the sagittal and coronal images for 3D accuracy.

Figure 4.19 Ultrasound probe attached to motor used for sagittal image acquisition for 3D reconstruction of the prostate and OAR. OAR, organs at risk.
Implant Techniques
In our institutions, we favor two interactive inversely planned approaches, which differ in whether isotopes are placed loosely or if they are custom linked in the OR. The first approach has been termed sagittally annealed vector evaluation (SAVE). In this technique, inversely planned simulated annealing (IPSA) is utilized to devise an intraoperative plan allowing for custom linked seed trains to be created in the OR and placed under image guidance with the needle vector overlaid on the live sagittal ultrasound image for precise placement of the linked seed train. This technique allows the brachytherapist the opportunity to link seeds together when extracapsular and seminal vesicle implants are desired. Our second approach is termed high dose rate emulated low dose rate prostate (HELP) brachytherapy (96), as needles are first placed and then the actual needle position is optimized utilizing IPSA to find the best isotope distribution within the already placed needle, much like a catheter when HDR implants are provided. This technique can be widely used during the implant of loose seeds, although the technique is often utilized in settings of prior radiation, TURPs, potential pubic arch interference, and times when mitigating factors make the knowledge of actual needle placement prior to isotope optimization beneficial.
Figure 4.20 Transverse, axial, and sagittal reconstruction of images acquired during automated sagittal imaging to the prostate and OAR. OAR, organs at risk.
“SAVE”: Sagittally Annealed Vector Evaluation
Linked-Seed Approach
In the traditional intraoperative planning approach, after the contours are completed, a treatment plan is generated in two steps. First, an inverse plan based on dose criteria to the contoured structures (prostate, urethra, and rectum) is generated using the simulated annealing optimization engine available in the planning software, which optimizes the needle and seed locations. The plan is then manually modified by the physicist and radiation oncologist to fully satisfy the required dose distribution goals (Figure 4.21). The metrics used for planning and evaluation are D90 > 140 Gy, V100% > 90%, V150% < 70%, and V200% < 30% for the prostate. The urethra goals are V150% = 0 cc and V125% < 1 cc. The rectal goal is V100% < 1 cc.
One additional degree of freedom for the planning process is offered by the use of linked seeds: the seeds and spacers are connected to each other during the assembly process and provide a semirigid linear structure (Figure 4.7). This enables all or part of the seed–spacer assembly outside of the prostate itself with reduced risk of seed migration. The option to place seeds more peripherally can permit better urethral sparing during the planning process (Figure 4.12).
After the plan is approved by the radiation oncologist, the seed–spacer trains are assembled by the physicist using the QUICKLINK (Bard Inc., Covington, GA) and inserted into needles, which are in turn housed in a shielded enclosure (Figures 4.8 and 4.14). Needles are inserted one by one under ultrasound guidance using the sagittal view. The software, under the direction of the physicist, helps guide the physician’s needle insertion by providing an outline of the planned needle location overlaid on the live ultrasound image (Figure 4.22). Once a needle is placed, seeds and spacers are manually deposited into the prostate and the needle is removed. The actual delivered needle location is updated in the treatment planning software after insertion. This sequential process of needle insertion and seed–spacer delivery is repeated for each needle until all planned needles are delivered. A new ultrasound image is then acquired on which the contours, seeds, and dose are overlaid. After verifying that the contours represent planned anatomy and the seeds are consistent with planned needle tracts, the dose is carefully reviewed to assess if the planning goals have been met. If there are areas of undercoverage, additional seeds can be inserted to fully achieve the dosimetric goals. Figure 4.23 shows the layout and working areas of the brachytherapy team within the OR.
Figure 4.21 Physician and physicists working together to create a plan meeting all implant metrics and criteria.

Figure 4.22 Image-guided needle placement utilizing real-time sagittal ultrasound guidance.
HELP Loose Seeds
In comparison, the HELP technique (96) differs in a few critical steps from the technique described earlier. After the radiation oncologist completes the contours, an ideal empty needle distribution is generated by the physician and physicist, which is similar to what might be done in an HDR procedure. The main criteria for the distribution are peripheral locations, adequate spacing, and enough potential seed locations for an optimum dose distribution. The needles are inserted by the radiation oncologist under sagittal ultrasound guidance and with the outline of the intended needle location overlaid on the live image. The actual needle position is not necessarily required to agree with the exact intended location. Rather, the software is simply updated to reflect the actual position of the empty needle (Figure 4.24). During this process the empty needle plan can be modified to account for the actual needle position and correct any imbalances in positioning. A second ultrasound scan is acquired with all needles inserted, on which the original prostate, urethra, and rectum contours are overlaid and modified by the radiation oncologist as needed. Just as in the standard technique, the treatment plan is generated in two steps; an inverse plan is generated using the simulated annealing IPSA engine and then modified manually. The only difference between the two techniques is that the seed distribution is optimized based on the actual needle locations already within the prostate (Figure 4.25). This treatment plan, with updated contours and needle position, provides the basis for the dosimetry on the day of treatment “d0 dosimetry” for this technique. After the radiation oncologist approves the treatment plan, the automated seed–spacer delivery takes place. A seed afterloader (SeedSelectronTM, Nucletron, an Elekta company, Elekta AB, Stockholm, Sweden) is used to build the seed–spacer sequence specific to each planned needle (Figure 4.26). The seeds and spacers, housed in cartridges, are placed inside the needle and pushed to the planned location by a drive wire. The afterloader also retracts the needle through a retraction mechanism independent of the drive wire, leaving the seeds and spacers (which are not linked to each other) inside the patient. A detailed description of the SeedSelectron is published elsewhere (97). This sequential process of needle insertion and seed–spacer delivery is repeated for each needle until all planned needles are delivered. In programs without a SeedSelectron the use of a Mick applicator (Mick Radionuclear Instruments, New York, NY) to deposit loose seeds can also be considered.
Figure 4.23 Layout and working areas of the brachytherapy team within the operating room.

Figure 4.24 In the HELP technique all of the needles are placed first in a peripheral loading pattern and updated real time to their actual position under live sagittal ultrasound imaging. The optimal isotope positions within the placed needles are then inversely planned. HELP, high dose rate emulated low dose rate prostate.
Figure 4.25 Inversely planned isodose distributions based on actual needle positions within the prostate.

Figure 4.26 The seed afterloader can receive loading instructions directly from the treatment-planning computer. Seeds and spacers are loaded within the needle according to the plan in the operating room. Each needle will be manually unloaded under direct ultrasound observation in the patient.
Dosimetric Constraints
Target
Postplan
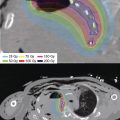
Posttreatment planning is used to confirm the dose delivered to the prostate as well as evaluate the dosimetry to the OAR. Postplan dosimetry can be performed immediately after the procedure, 1 day postoperatively, or up to 1 month from the date of implant. The ABS recommends that all implants be evaluated with CT-based dosimetry. The feasibility of ultrasound and MRI-based assessment is also being explored. The optimal time for postimplant dosimetry is isotope dependent based on the influence of half-life and expected postimplant edema (98). For 125I and 103Pd, assessment is typically performed at 30 ± 7 days and 16 ± 4 days, respectively. For 125I, many brachytherapist wait for 4 weeks to allow for maximal resolution of inflammation and edema associated with the implant. However, it can be done earlier, with the realization that the final dosimetry would likely improve at a later date when swelling resolves. Postimplant dosimetry with ultrasound is difficult because the artifact from the seeds causes image degradation; however, the ultrasound should be included in conjunction with the CT scan for dosimetric assessment of the implant. The ultrasound not only confirms what was the target at the time of the implant, it also helps to verify the length of the prostate to help define the apex of the prostate, which at times can be difficult on CT. MRI can be used as an excellent way to define prostatic anatomy; however, void artifacts from the seeds (Figure 4.27) make seed identification and subsequent dosimetry difficult. Newer MRI protocols allow for greater seed suppression, although voids are still apparent. CT scans allow visualization of the permanent seeds; however, the prostate anatomy is difficult to define due to seed artifact (99) (Figure 4.28). Image fusion between CT scan and MRI used to evaluate the prostate can also be applied for dosimetry (100) and evaluation of prostatic swelling (101). In our practice we often pair a CT scan and MRI scan to allow for precise isotope location on the CT scan and clear prostate anatomy on the MRI scan (Figure 4.29). Data from the ultrasound at the time of implant help to further refine the clinical assessment of the implant. Catheterization at the time of CT allows for better visualization of the urethra.
Dosimetric data depend on the contours drawn to represent the postimplant prostate and OAR. To evaluate coverage of the target, the dose to 90% of prostate (D90) is calculated. Implants with a D90 > 90% of the prescribed dose or alternatively 140 Gy for 125I (102) have been shown to have better PSA relapse-free outcomes (103). Interobserver variation in contouring the prostate based on CT images varies greatly; therefore, it is important to have a standard methodology for contouring the prostate. With CT-based dosimetry, the temptation to circle seeds can be great. To help minimize this tendency, utilizing data from the TRUS volume study and/or an MRI scan can greatly improve the quality of implant assessment. The ABS recommends the reporting of target D90, D80, D100, V80, V90, V100, V150, and V200, as well as rectal and urethral doses (13).

Figure 4.27 Seed void artifacts seen on MRI scan.
Figure 4.28 Seeds clearly identified on CT scan.

Figure 4.29 MR–CT fusion for Day 30 dosimetric assessment of the implant.
Organs at Risk
The rectum, urethra, penile bulb, and neurovascular bundles are the normal structures in close proximity to the prostate and are within the high-dose region of the prostate implant. Dosimetry to the OAR needs to be reported to better understand dose and volume constraints and their relation to acute and long-term toxicity. Crook et al highlight the importance of standard dosimetry reporting of the OAR so that data from different centers can be combined to establish dosimetric guidelines for the OAR. They recommend that rectal wall contours be performed on all CT scan slices where seeds can be visualized and the V100 and V150 be reported. The whole urethral volume should be contoured rather than points or a representative volume; the urethral V150, D5, and D30 should be recorded (104). Contouring of the penile bulb and neurovascular bundles as well as evaluation of dose to these structures requires further study, although penile bulb dose has been associated with erectile function (105).