Chapter FOUR
Genitourinary Imaging
KELLY S.FREED
HISTORY
Patient A: A 31-year-old woman who had been in a motor vehicle accident presents with gross hematuria. Patient B: A 21-year-old woman who also had been in a motor vehicle accident presents with microscopic hematuria.
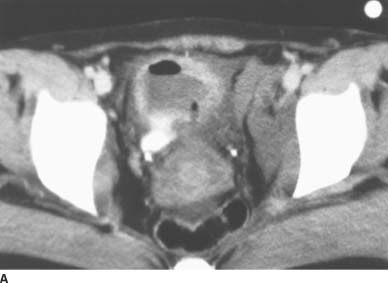
 FIGURE 4-1A Patient A: Contrast-enhanced CT through the bladder. Extravasation of contrast material is seen from the right lateral aspect of the urinary bladder. The bladder is not well distended, and it is difficult to determine if the rupture is intraperitoneal or extraperitoneal. A CT cystogram or conventional cystogram could be performed to clarify this issue.
FIGURE 4-1A Patient A: Contrast-enhanced CT through the bladder. Extravasation of contrast material is seen from the right lateral aspect of the urinary bladder. The bladder is not well distended, and it is difficult to determine if the rupture is intraperitoneal or extraperitoneal. A CT cystogram or conventional cystogram could be performed to clarify this issue.
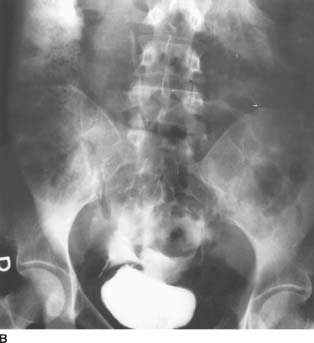
 FIGURE 4-1B Patient A: Anteroposterior plain film of the abdomen performed following the contrast-enhanced CT scan. Contrast material is seen superior to the bladder, tracking up along the right paracolic gutter and adjacent to the liver.
FIGURE 4-1B Patient A: Anteroposterior plain film of the abdomen performed following the contrast-enhanced CT scan. Contrast material is seen superior to the bladder, tracking up along the right paracolic gutter and adjacent to the liver.
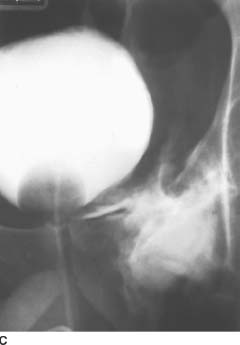
 FIGURE 4-1C Patient B: Spot film from a conventional cystogram via a Foley catheter. There is a fracture of the left inferior pubic ramus. Contrast material is extravasating into the left inguinal region.
FIGURE 4-1C Patient B: Spot film from a conventional cystogram via a Foley catheter. There is a fracture of the left inferior pubic ramus. Contrast material is extravasating into the left inguinal region.
 The differential diagnosis for both patients includes intraperitoneal bladder rupture, extraperitoneal bladder rupture, or a combination of the two.
The differential diagnosis for both patients includes intraperitoneal bladder rupture, extraperitoneal bladder rupture, or a combination of the two.
 In Patient A, intraperitoneal rupture is diagnosed because the contrast outlines the right paracolic gutter and liver.
In Patient A, intraperitoneal rupture is diagnosed because the contrast outlines the right paracolic gutter and liver.
 In Patient B, extraperitoneal rupture is diagnosed because the contrast material does not flow into the peritoneal cavity but extends into the proximal thigh via the left inguinal region. There is an associated fracture of the inferior pubic ramus.
In Patient B, extraperitoneal rupture is diagnosed because the contrast material does not flow into the peritoneal cavity but extends into the proximal thigh via the left inguinal region. There is an associated fracture of the inferior pubic ramus.
DIAGNOSIS
Patient A: Intraperitoneal bladder rupture Patient B: Extraperitoneal bladder rupture
KEY FACTS
Clinical
 Bladder rupture can be seen following blunt or penetrating trauma and may be extraperitoneal, intraperitoneal, or both.
Bladder rupture can be seen following blunt or penetrating trauma and may be extraperitoneal, intraperitoneal, or both.
 Extraperitoneal bladder rupture is more common, composing approximately 80% of cases, and is frequently associated with pelvic fractures. The rupture usually occurs at the base of the bladder. Intraperitoneal bladder rupture occurs at the dome of the bladder when the bladder is distended. Pelvic fractures are seen less commonly in intraperitoneal bladder rupture than in extraperitoneal bladder rupture.
Extraperitoneal bladder rupture is more common, composing approximately 80% of cases, and is frequently associated with pelvic fractures. The rupture usually occurs at the base of the bladder. Intraperitoneal bladder rupture occurs at the dome of the bladder when the bladder is distended. Pelvic fractures are seen less commonly in intraperitoneal bladder rupture than in extraperitoneal bladder rupture.
 Intraperitoneal rupture is more common in children than adults.
Intraperitoneal rupture is more common in children than adults.
 Physical findings of bladder rupture include hematuria and the inability to urinate. Significant hematuria (> 50 red blood cells/high-power field) is a sensitive indicator of bladder trauma.
Physical findings of bladder rupture include hematuria and the inability to urinate. Significant hematuria (> 50 red blood cells/high-power field) is a sensitive indicator of bladder trauma.
 Intraperitoneal bladder rupture requires surgery with bladder closure, whereas extraperitoneal bladder rupture can be managed with catheter drainage, antibiotics, and clinical follow-up.
Intraperitoneal bladder rupture requires surgery with bladder closure, whereas extraperitoneal bladder rupture can be managed with catheter drainage, antibiotics, and clinical follow-up.
Radiologic
 Radiologic diagnosis includes conventional cystography and CT of the abdomen and pelvis, including CT cystography.
Radiologic diagnosis includes conventional cystography and CT of the abdomen and pelvis, including CT cystography.
 In extraperitoneal rupture, there are often associated fractures of the pubic rami or anterior pelvic ring. The extravasated contrast material can track down into the proximal thigh or scrotum. The extravasated contrast material may be ill-defined and feathery or contained.
In extraperitoneal rupture, there are often associated fractures of the pubic rami or anterior pelvic ring. The extravasated contrast material can track down into the proximal thigh or scrotum. The extravasated contrast material may be ill-defined and feathery or contained.
 With intraperitoneal rupture, the contrast material flows freely into the peritoneum and may outline bowel loops or the paracolic gutters.
With intraperitoneal rupture, the contrast material flows freely into the peritoneum and may outline bowel loops or the paracolic gutters.
 CT of the abdomen and pelvis performed with the bladder only mildly or moderately distended is not as sensitive as conventional cystography for bladder injury. However, recent articles have demonstrated that CT cystography is comparably sensitive to conventional cystography. The bladder must be well distended on the CT study, either from instillation of contrast material through a Foley catheter or by using delayed images. Postdrainage CT images should also be obtained.
CT of the abdomen and pelvis performed with the bladder only mildly or moderately distended is not as sensitive as conventional cystography for bladder injury. However, recent articles have demonstrated that CT cystography is comparably sensitive to conventional cystography. The bladder must be well distended on the CT study, either from instillation of contrast material through a Foley catheter or by using delayed images. Postdrainage CT images should also be obtained.
SUGGESTED READING
Bodner DR, Selzman AA, Spirnak JP. Evaluation and treatment of bladder rupture. Semin Urol 1995;13:62–65.
Chan DP, Abujudeh HH, Cushing GL Jr, Novelline RA. CT cystography with multiplanar reformation for suspected bladder rupture: Experience in 234 cases. AJR Am J Roentgenol 2006;187:1296–302.
Horstman WG, McClennan BL, Heiken JP. Comparison of computed tomography and conventional cystography for detection of traumatic bladder rupture. Urol Radiol 1991;12:188–193.
Morey AF, Iverson AJ, Swan A, et al. Bladder rupture after blunt trauma: guidelines for diagnostic imaging. J Trauma 2001;51:683–686.
Rehm CG, Mure AJ, O’Malley KF, Ross SE. Blunt traumatic bladder rupture: The role of retrograde cystogram. Ann Emerg Med 1991;20:845–847.
MARY T. KEOGAN
HISTORY
A 56-year-old woman presents with a low-grade fever.
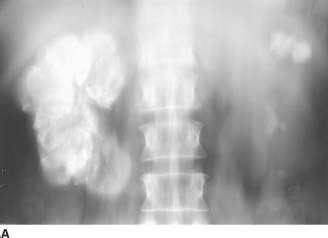
 FIGURE 4-2A Noncontrast linear coronal tomogram of the renal area. Extensive calcifications are noted in the right kidney. Calcifications are also noted in the upper and lower pole calyces of the left kidney.
FIGURE 4-2A Noncontrast linear coronal tomogram of the renal area. Extensive calcifications are noted in the right kidney. Calcifications are also noted in the upper and lower pole calyces of the left kidney.
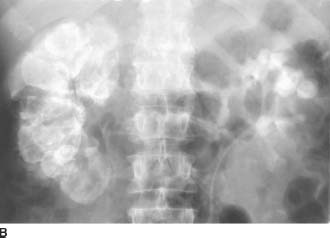
 FIGURE 4-2B Five-minute anteroposterior view of the kidneys during an intravenous urogram. There is no excretion of contrast material from the right kidney. Calcifications are noted in the right proximal ureter. The left upper pole calyces are dilated, and there is no filling of the left lower pole calyces.
FIGURE 4-2B Five-minute anteroposterior view of the kidneys during an intravenous urogram. There is no excretion of contrast material from the right kidney. Calcifications are noted in the right proximal ureter. The left upper pole calyces are dilated, and there is no filling of the left lower pole calyces.
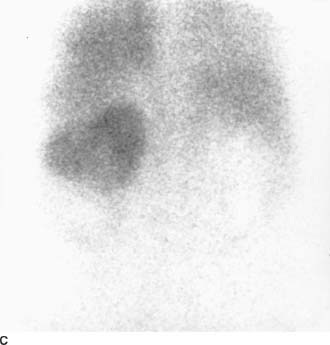
 FIGURE 4-2C Tc99m-diethylenetriamine penta-acetic acid scan of the renal area in the posteroanterior projection. There is marked reduction of activity in the left lower pole, with a faint rim of cortical activity. No right renal activity is seen.
FIGURE 4-2C Tc99m-diethylenetriamine penta-acetic acid scan of the renal area in the posteroanterior projection. There is marked reduction of activity in the left lower pole, with a faint rim of cortical activity. No right renal activity is seen.
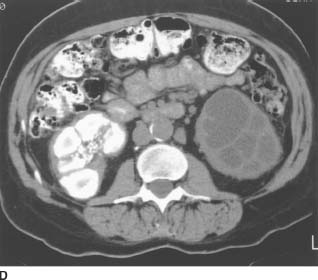
 FIGURE 4-2D Noncontrast CT of the lower abdomen. There are dense calcifications in the right kidney and ureter. Low-attenuation areas are noted in the left lower pole consistent with a dilated collecting system.
FIGURE 4-2D Noncontrast CT of the lower abdomen. There are dense calcifications in the right kidney and ureter. Low-attenuation areas are noted in the left lower pole consistent with a dilated collecting system.
 Renal tuberculosis (TB): This is the best diagnosis due to extensive replacement of the nonfunctioning right kidney by calcification, the so called putty kidney. Calcifications in the ureter are also typical. Pyonephrosis is present in the lower pole of the left kidney.
Renal tuberculosis (TB): This is the best diagnosis due to extensive replacement of the nonfunctioning right kidney by calcification, the so called putty kidney. Calcifications in the ureter are also typical. Pyonephrosis is present in the lower pole of the left kidney.
 Xantogranulomatosis pyelonephritis (XGP): This inflammatory process is usually related to a chronic urinary obstruction and a staghorn calculus. Calcification in the ureter is atypical.
Xantogranulomatosis pyelonephritis (XGP): This inflammatory process is usually related to a chronic urinary obstruction and a staghorn calculus. Calcification in the ureter is atypical.
 Schistosomiasis: This infection characteristically affects the distal ureters, causing dilation and/or stenosis. The proximal ureters and ureteropelvic junctions are rarely involved. Schistosomiasis typically causes bladder calcification, but renal calcifications are uncommon.
Schistosomiasis: This infection characteristically affects the distal ureters, causing dilation and/or stenosis. The proximal ureters and ureteropelvic junctions are rarely involved. Schistosomiasis typically causes bladder calcification, but renal calcifications are uncommon.
DIAGNOSIS
TB with right autonephrectomy and left lower pole pyonephrosis
KEY FACTS
Clinical
 Renal TB results from hematogenous spread of TB bacilli to the kidneys. Ureteral involvement is secondary to bacilluria from the kidneys.
Renal TB results from hematogenous spread of TB bacilli to the kidneys. Ureteral involvement is secondary to bacilluria from the kidneys.
 Although both kidneys are usually involved, the disease process is typically more severe in one kidney.
Although both kidneys are usually involved, the disease process is typically more severe in one kidney.
 Patients are typically >40 years and present with hematuria, frequency, dysuria, or suprapubic pain.
Patients are typically >40 years and present with hematuria, frequency, dysuria, or suprapubic pain.
 About 10% of patients may be asymptomatic and have sterile urine.
About 10% of patients may be asymptomatic and have sterile urine.
Radiologic
 Radiographic findings depend on the extent of the disease process but are present in the majority of cases of renal TB.
Radiographic findings depend on the extent of the disease process but are present in the majority of cases of renal TB.
 Papillary necrosis is common and may be extensive. Necrosis in renal granulomas may lead to the formation of communicating cavities.
Papillary necrosis is common and may be extensive. Necrosis in renal granulomas may lead to the formation of communicating cavities.
 Parenchymal calcifications are present in 50% of patients. They may be amorphous in association with granulomatous masses, or dense in healed tuberculomas. Renal calculi develop in 20% of patients.
Parenchymal calcifications are present in 50% of patients. They may be amorphous in association with granulomatous masses, or dense in healed tuberculomas. Renal calculi develop in 20% of patients.
 Parenchymal scarring occurs in 20% of patients, either localized or involving the entire kidney. There are also associated calcifications and underlying calyceal abnormalities.
Parenchymal scarring occurs in 20% of patients, either localized or involving the entire kidney. There are also associated calcifications and underlying calyceal abnormalities.
 Calyceal abnormalities are common, with multiple irregular strictures of the infundibula and subsequent hydrocalycosis.
Calyceal abnormalities are common, with multiple irregular strictures of the infundibula and subsequent hydrocalycosis.
 Renal function is impaired in 50% of patients. Antegrade or retrograde pyelography is often required for evaluation.
Renal function is impaired in 50% of patients. Antegrade or retrograde pyelography is often required for evaluation.
 Advanced disease eventually results in a nonfunctioning kidney (autonephrectomy or “putty” kidney). These cases are associated with extensive calcifications.
Advanced disease eventually results in a nonfunctioning kidney (autonephrectomy or “putty” kidney). These cases are associated with extensive calcifications.
 Failure of contrast material excretion often signifies the presence of tuberculous pyonephrosis due to stricture formation.
Failure of contrast material excretion often signifies the presence of tuberculous pyonephrosis due to stricture formation.
 Abnormalities of the ureters occur in 50% of cases of renal TB due to ulceration, with fibrosis, stricture, and calcification. Alternating segments of dilation and stricture produce a characteristic beaded appearance. Shortening of the ureter may also occur, producing a “pipestem” appearance.
Abnormalities of the ureters occur in 50% of cases of renal TB due to ulceration, with fibrosis, stricture, and calcification. Alternating segments of dilation and stricture produce a characteristic beaded appearance. Shortening of the ureter may also occur, producing a “pipestem” appearance.
 Other sites of urinary tract involvement include the prostate, epididymis, scrotum, and bladder, producing calcification with abscess formation and fistulous tracts.
Other sites of urinary tract involvement include the prostate, epididymis, scrotum, and bladder, producing calcification with abscess formation and fistulous tracts.
SUGGESTED READING
Renal inflammatory disease. In NR Dunnick, RW McCallum, CM Sandler (eds), Textbook of Uroradiology. Baltimore: Williams & Wilkins, 1991;135–157.
The ureter. In NR Dunnick, RW McCallum, CM Sandler (eds), Textbook of Uroradiology. Baltimore: Williams & Wilkins, 1991;287–319.
Craig WD, Wagner BJ, Travis MD. Pyelonephritis: Radiologic-pathologic review. Radiographics 2008;28:255–277.
Elkin M. Urogenital tuberculosis. In HM Pollock, H Elkin (eds), Clinical Urography. Philadelphia: Saunders, 1990;1020–1052.
Paterson A. Urinary tract infection: An update on imaging strategies. Eur Radiol 2004;14(Suppl 4):L89–L100.
ANTHONY M. FOTI
HISTORY
A 50-year-old man has back pain and an elevated serum creatinine.
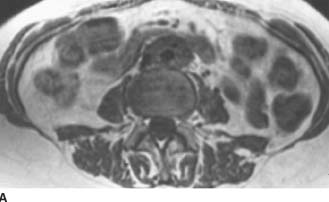
 FIGURE 4-3A Axial T1-weighted MRI of the abdomen. There is a well-circumscribed mantle of soft tissue surrounding the aorta and abutting the IVC. The mass does not displace the aorta anteriorly away from the spine and is isointense to muscle.
FIGURE 4-3A Axial T1-weighted MRI of the abdomen. There is a well-circumscribed mantle of soft tissue surrounding the aorta and abutting the IVC. The mass does not displace the aorta anteriorly away from the spine and is isointense to muscle.
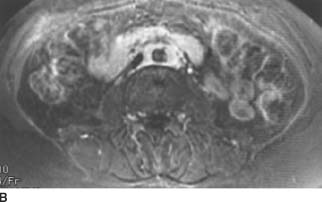
 FIGURE 4-3B Axial fat-suppressed T2-weighted MRI of the abdomen. The mantle of soft tissue is mildly hyperintense and relatively homogenous.
FIGURE 4-3B Axial fat-suppressed T2-weighted MRI of the abdomen. The mantle of soft tissue is mildly hyperintense and relatively homogenous.
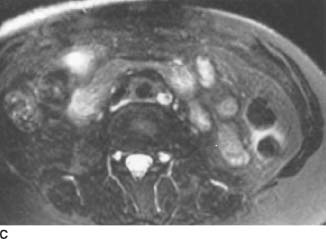
 FIGURE 4-3C Axial contrast-enhanced T1-weighted MRI following the intravenous administration of a gadolinium-chelate. There is uniform enhancement of the soft tissue mass.
FIGURE 4-3C Axial contrast-enhanced T1-weighted MRI following the intravenous administration of a gadolinium-chelate. There is uniform enhancement of the soft tissue mass.
 Malignant retroperitoneal fibrosis (RPF): Imaging cannot differentiate malignant from nonmalignant RPF reliably; however, malignant RPF tends to be more heterogeneous on T2-weighted MR images.
Malignant retroperitoneal fibrosis (RPF): Imaging cannot differentiate malignant from nonmalignant RPF reliably; however, malignant RPF tends to be more heterogeneous on T2-weighted MR images.
 Malignant lymphadenopathy and lymphoma: These entities tend to displace the aorta anteriorly, away from the spine.
Malignant lymphadenopathy and lymphoma: These entities tend to displace the aorta anteriorly, away from the spine.
 Idiopathic RPF: The periaortic distribution and signal characteristics are classic for this entity.
Idiopathic RPF: The periaortic distribution and signal characteristics are classic for this entity.
DIAGNOSIS
Idiopathic (nonmalignant) RPF
KEY FACTS
Clinical
 RPF is a rare disorder in which a fibrotic plaque encases the aorta and extends laterally to engulf the inferior vena cava (IVC) and ureters. It usually begins near the aortic bifurcation and extends cephalad to the renal hila. Occasionally, it may extend cranially into the mediastinum or anteriorly into the mesentery.
RPF is a rare disorder in which a fibrotic plaque encases the aorta and extends laterally to engulf the inferior vena cava (IVC) and ureters. It usually begins near the aortic bifurcation and extends cephalad to the renal hila. Occasionally, it may extend cranially into the mediastinum or anteriorly into the mesentery.
 70% of patients are 30 to 60 years of age at the time of diagnosis.
70% of patients are 30 to 60 years of age at the time of diagnosis.
 Symptoms are nonspecific and include dull back pain, fatigue, and weight loss. Laboratory values include elevated serum creatinine levels and erythrocyte sedimentation rates.
Symptoms are nonspecific and include dull back pain, fatigue, and weight loss. Laboratory values include elevated serum creatinine levels and erythrocyte sedimentation rates.
 Two-thirds of cases are idiopathic (Ormond’s disease). The presumed mechanism is autoimmune, likely a response to leakage of ceroid, an insoluble lipid, from atherosclerotic plaques into periaortic tissue. Twelve percent of cases are secondary to methysergide administration; beta blockers, hydralazine, methyldopa, and bromocriptine have also been implicated. Other causes include malignancy, hemorrhage, and aneurysms (so-called perianeurysmal fibrosis).
Two-thirds of cases are idiopathic (Ormond’s disease). The presumed mechanism is autoimmune, likely a response to leakage of ceroid, an insoluble lipid, from atherosclerotic plaques into periaortic tissue. Twelve percent of cases are secondary to methysergide administration; beta blockers, hydralazine, methyldopa, and bromocriptine have also been implicated. Other causes include malignancy, hemorrhage, and aneurysms (so-called perianeurysmal fibrosis).
 Malignant RPF is an intense desmoplastic response to retroperitoneal metastases from a variety of primary malignancies (breast, lung, thyroid, gastrointestinal tract, genitourinary tract, and Hodgkin’s lymphoma). There are only scattered malignant cells, and thus deep surgical biopsy is required to differentiate nonmalignant from malignant RPF.
Malignant RPF is an intense desmoplastic response to retroperitoneal metastases from a variety of primary malignancies (breast, lung, thyroid, gastrointestinal tract, genitourinary tract, and Hodgkin’s lymphoma). There are only scattered malignant cells, and thus deep surgical biopsy is required to differentiate nonmalignant from malignant RPF.
 Histologically, perianeurysmal fibrosis (also referred to as an inflammatory aneurysm) is identical to RPF. The only difference is the caliber of the aorta.
Histologically, perianeurysmal fibrosis (also referred to as an inflammatory aneurysm) is identical to RPF. The only difference is the caliber of the aorta.
 RPF usually results in ureteral dilatation by impairing peristalsis, rather than directly invading the ureter.
RPF usually results in ureteral dilatation by impairing peristalsis, rather than directly invading the ureter.
 RPF may obstruct the IVC and, rarely, the portal vein or common bile duct.
RPF may obstruct the IVC and, rarely, the portal vein or common bile duct.
 Treatment consists of a combination of surgery to release the ureters (ureterolysis) and steroids.
Treatment consists of a combination of surgery to release the ureters (ureterolysis) and steroids.
 RPF has a similar histology and is associated with other systemic sclerosing diseases, including sclerosing cholangitis, orbital pseudotumor, mediastinal fibrosis, and Riedel’s thyroiditis. There is also an association with other immune-mediated connective tissue disorders such as ankylosing spondylitis, Wegener’s granulomatosis, systemic lupus erythematosus, Raynaud’s disease, polyarteritis nodosa, and systemic vasculitis. RPF is associated with the major histocompatibility complex HLA-B27.
RPF has a similar histology and is associated with other systemic sclerosing diseases, including sclerosing cholangitis, orbital pseudotumor, mediastinal fibrosis, and Riedel’s thyroiditis. There is also an association with other immune-mediated connective tissue disorders such as ankylosing spondylitis, Wegener’s granulomatosis, systemic lupus erythematosus, Raynaud’s disease, polyarteritis nodosa, and systemic vasculitis. RPF is associated with the major histocompatibility complex HLA-B27.
Radiologic
 On intravenous urography there is hydronephrosis with medial deviation of the middle third of the ureters, which then taper near the L4 to L5 level. This is in contradistinction to most cases of lymphoma and other causes of lymphadenopathy, which are not associated with a desmoplastic response and thus cause lateral deviation of the ureters due to mass effect.
On intravenous urography there is hydronephrosis with medial deviation of the middle third of the ureters, which then taper near the L4 to L5 level. This is in contradistinction to most cases of lymphoma and other causes of lymphadenopathy, which are not associated with a desmoplastic response and thus cause lateral deviation of the ureters due to mass effect.
 On CT, a homogeneous mantle of soft tissue envelopes, but usually does not displace—the aorta. It extends laterally to involve the IVC and ureters, but usually does not extend >1 cm lateral to the ureters. It may obstruct the gonadal vessels. The margins are usually sharply circumscribed and not nodular. However, it may be ill-defined, although the margin characteristics cannot be used to distinguish nonmalignant from malignant RPF reliably. Precontrast, it is isoattenuating with the psoas muscles. Postcontrast, the soft tissue mass enhances uniformly, although enhancement diminishes with the chronicity of the disease.
On CT, a homogeneous mantle of soft tissue envelopes, but usually does not displace—the aorta. It extends laterally to involve the IVC and ureters, but usually does not extend >1 cm lateral to the ureters. It may obstruct the gonadal vessels. The margins are usually sharply circumscribed and not nodular. However, it may be ill-defined, although the margin characteristics cannot be used to distinguish nonmalignant from malignant RPF reliably. Precontrast, it is isoattenuating with the psoas muscles. Postcontrast, the soft tissue mass enhances uniformly, although enhancement diminishes with the chronicity of the disease.
 On ultrasound, a homogeneous hypoechoic perivascular mantle is characteristic.
On ultrasound, a homogeneous hypoechoic perivascular mantle is characteristic.
 With MRI, the soft tissue mass is relatively homogeneous on all imaging sequences. It is isointense to psoas muscle on Tl-weighted images. The signal intensity on T2-weighted images and the enhancement on Tl-weighted images post-gadolinium-chelate administration vary with the stage. Early in the disease, the cellular nature of the infiltrate results in high signal intensity on T2-weighted images and discernible contrast enhancement. Late in the disease, the signal intensity on T2-weighted images and contrast enhancement decreases, reflecting the fibrotic process. Heterogeneous high signal intensity on T2-weighted images suggests malignancy, while uniformly low signal suggests late-stage benign disease.
With MRI, the soft tissue mass is relatively homogeneous on all imaging sequences. It is isointense to psoas muscle on Tl-weighted images. The signal intensity on T2-weighted images and the enhancement on Tl-weighted images post-gadolinium-chelate administration vary with the stage. Early in the disease, the cellular nature of the infiltrate results in high signal intensity on T2-weighted images and discernible contrast enhancement. Late in the disease, the signal intensity on T2-weighted images and contrast enhancement decreases, reflecting the fibrotic process. Heterogeneous high signal intensity on T2-weighted images suggests malignancy, while uniformly low signal suggests late-stage benign disease.
SUGGESTED READING
Amis ES Jr. Retroperitoneal fibrosis. AJR Am J Roentgenol 1991;157: 321-329.
Arrive L, Hricak H, Tavares NJ, Miller TR. Malignant versus nonmalignant retroperitoneal fibrosis: differentiation with MR imaging. Radiology 1989;172:139–143.
Brooks AP, Reznek RH, Webb JA. Aortic displacement on computed tomography of idiopathic retroperitoneal fibrosis. Clin Radiol 1989;40:51–52.
Brun B, Laursen K, Sorensen IN, et al. CT in retroperitoneal fibrosis. AJR Am J Roentgenol 1981;137:535–538.
Cronin CG, Lohan DG, Blake MA, et al. Retroperitoneal fibrosis: a review of clinical features and imaging findings. AJR Am J Roentgenol 2008;191:423–431.
Degesys GE, Dunnick NR, Silverman PM, et al. Retroperitoneal fibrosis: use of CT in distinguishing among possible causes. AJR Am J Roentgenol 1986;146:57–60.
Kottra JJ, Dunnick NR. Retroperitoneal fibrosis. Radiol Clin North Am 1996;34:1259–1275.
JEREMY A.L.
LAWRANCE
HISTORY
A 67-year-old woman who was previously healthy presents with a 6-week history of epigastric pain.
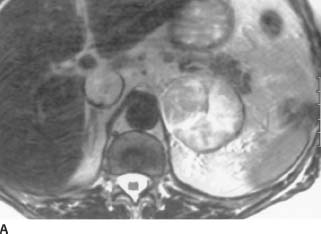
 FIGURE 4-4A Axial T2-weighted MRI of the upper abdomen. There is a 5-cm left-upper quadrant mass. It is of relatively high signal intensity and heterogeneous in nature. In addition, a highsignal intensity mass is noted in the IVC.
FIGURE 4-4A Axial T2-weighted MRI of the upper abdomen. There is a 5-cm left-upper quadrant mass. It is of relatively high signal intensity and heterogeneous in nature. In addition, a highsignal intensity mass is noted in the IVC.
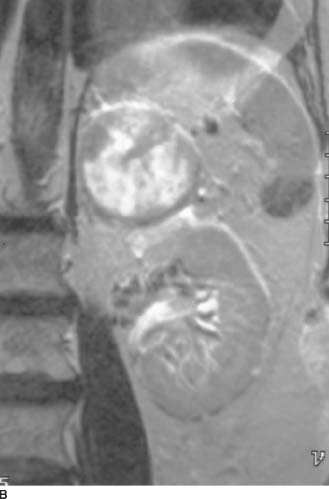
 FIGURE 4-4B Coronal T2-weighted MRI of the upper abdomen. The mass is superior to and clearly separate from the left kidney.
FIGURE 4-4B Coronal T2-weighted MRI of the upper abdomen. The mass is superior to and clearly separate from the left kidney.
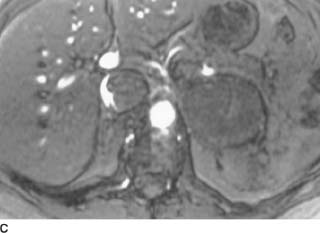
 FIGURE 4-4C Axial T1-weighted gradient echo MRI of the upper abdomen shows a crescent of flow around the IVC filling defect.
FIGURE 4-4C Axial T1-weighted gradient echo MRI of the upper abdomen shows a crescent of flow around the IVC filling defect.
 Renal cell carcinoma: The mass is separate from the kidney; therefore, this diagnosis is excluded.
Renal cell carcinoma: The mass is separate from the kidney; therefore, this diagnosis is excluded.
 Pheochromocytoma: These tumors usually demonstrate very high signal on T2-weighted MR images. They are usually > 3 cm and are frequently necrotic and hemorrhagic. The IVC invasion, however, is not a feature of pheochromocytomas.
Pheochromocytoma: These tumors usually demonstrate very high signal on T2-weighted MR images. They are usually > 3 cm and are frequently necrotic and hemorrhagic. The IVC invasion, however, is not a feature of pheochromocytomas.
 Adrenal metastasis: Tumors > 5 cm are more likely malignant. Furthermore, metastases are usually of higher signal intensity than adenomas. Apart from an adrenal metastasis in a patient with renal cell carcinoma, IVC invasion by an adrenal metastasis would be uncommon. The kidneys show no evidence of tumor in this case.
Adrenal metastasis: Tumors > 5 cm are more likely malignant. Furthermore, metastases are usually of higher signal intensity than adenomas. Apart from an adrenal metastasis in a patient with renal cell carcinoma, IVC invasion by an adrenal metastasis would be uncommon. The kidneys show no evidence of tumor in this case.
 Adrenal adenoma: This diagnosis is extremely unlikely unless IVC thrombosis is coincidental.
Adrenal adenoma: This diagnosis is extremely unlikely unless IVC thrombosis is coincidental.
 Adrenal carcinoma: Although these tumors typically are larger at presentation, direct IVC invasion makes this the most likely diagnosis.
Adrenal carcinoma: Although these tumors typically are larger at presentation, direct IVC invasion makes this the most likely diagnosis.
DIAGNOSIS
Adrenal carcinoma with inferior vena cava invasion
KEY FACTS
Clinical
 Adrenal carcinomas are rare malignant tumors with an annual incidence of 0.5 to 2.0 cases per million per year.
Adrenal carcinomas are rare malignant tumors with an annual incidence of 0.5 to 2.0 cases per million per year.
 The average age in one large study was 47 years.
The average age in one large study was 47 years.
 There is a slight female preponderance.
There is a slight female preponderance.
 In a series of 156 cases, 53% had a functional endocrine syndrome. Cushing’s syndrome is the most common, with virilization, hypertension, and feminization occurring less frequently.
In a series of 156 cases, 53% had a functional endocrine syndrome. Cushing’s syndrome is the most common, with virilization, hypertension, and feminization occurring less frequently.
 Up to 5% of cases are bilateral.
Up to 5% of cases are bilateral.
Radiologic
 Adrenal carcinomas tend to be large at presentation, usually > 5 cm in diameter. Functional tumors tend to be smaller at presention than nonfunctioning tumors. The range of sizes in one study was 3 to 30 cm, with a mean diameter of 12 cm.
Adrenal carcinomas tend to be large at presentation, usually > 5 cm in diameter. Functional tumors tend to be smaller at presention than nonfunctioning tumors. The range of sizes in one study was 3 to 30 cm, with a mean diameter of 12 cm.
 The problem with small adrenal carcinomas is that it is often impossible to differentiate benign from malignant tumors. Tumors > 5 cm are more likely malignant, while evidence of local invasion into adjacent organs or distant metastases are features of malignant tumors.
The problem with small adrenal carcinomas is that it is often impossible to differentiate benign from malignant tumors. Tumors > 5 cm are more likely malignant, while evidence of local invasion into adjacent organs or distant metastases are features of malignant tumors.
 In recent studies, metastases from adrenal carcinoma were present in 22%, while older studies reported higher incidences of metastases. The most common sites are liver, lymph nodes, bone, and lungs.
In recent studies, metastases from adrenal carcinoma were present in 22%, while older studies reported higher incidences of metastases. The most common sites are liver, lymph nodes, bone, and lungs.
 Areas of necrosis, hemorrhage, and calcification are common. The latter is best detected by CT and found in approximately 30% of cases.
Areas of necrosis, hemorrhage, and calcification are common. The latter is best detected by CT and found in approximately 30% of cases.
 By MRI, adrenal carcinoma shows low signal intensity on T1-weighted images and signal intensity greater than liver on T2-weighted images. Pheochromocytomas tend to have very high signal intensity on T2-weighted images and can be difficult to distinguish from adrenal carcinomas with MRI. Detection and delineation of vascular invasion, as well as multiplanar capability, make MRI a useful diagnostic tool in cases of adrenal carcinoma.
By MRI, adrenal carcinoma shows low signal intensity on T1-weighted images and signal intensity greater than liver on T2-weighted images. Pheochromocytomas tend to have very high signal intensity on T2-weighted images and can be difficult to distinguish from adrenal carcinomas with MRI. Detection and delineation of vascular invasion, as well as multiplanar capability, make MRI a useful diagnostic tool in cases of adrenal carcinoma.
SUGGESTED READING
Dunnick NR. Adrenal carcinoma. Radiol Clin North Am 1994;32:99–108.
Icard P, Chapuis Y, Andreassian B, et al. Adrenocortical carcinoma in surgically treated patients: a retrospective study on 156 cases. French Assoc Endocrine Surg 1992;112:972–980.
Johnson PT, Horton KM, Fishman EK. Adrenal mass imaging with multi-detector CT: pathologic conditions, pearls, and pitfalls. Radiographics 2009;29:1333–1351.
Slattery JM, Blake MA, Kalra MK, et al. Adrenocortical carcinoma: Contrast washout characteristics on CT. AJR Am J Roentgenol 2006;187:W21-W24.
Zografos GC, Driscoll DL, Karakousis CP, Humen RP. Adrenal adenocarcinoma: a review of 53 cases. Surg Oncol 1994;55:160–164.
RICHARD A.
LEDER
HISTORY
A 46-year-old man presents with urinary frequency.
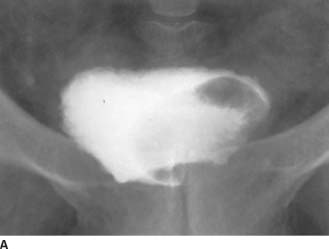
 FIGURE 4-5A Anteroposterior supine 10-minute film of the bladder from an intravenous urogram demonstrates an oval to round filling defect along the left side of the bladder with no evidence of ureteral obstruction on that side.
FIGURE 4-5A Anteroposterior supine 10-minute film of the bladder from an intravenous urogram demonstrates an oval to round filling defect along the left side of the bladder with no evidence of ureteral obstruction on that side.
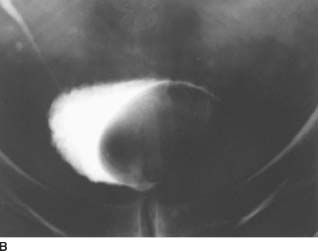
 FIGURE 4-5B Anteroposterior postvoid film from the same intravenous urogram shows a smooth filling defect involving the left lateral wall of the bladder.
FIGURE 4-5B Anteroposterior postvoid film from the same intravenous urogram shows a smooth filling defect involving the left lateral wall of the bladder.
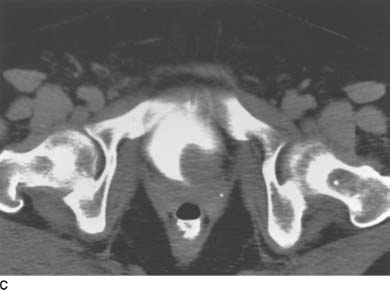
 FIGURE 4-5C Contrast-enhanced CT through the urinary bladder shows a smooth, round filling defect in the left posterolateral wall of the bladder. The filling defect is of soft tissue attenuation.
FIGURE 4-5C Contrast-enhanced CT through the urinary bladder shows a smooth, round filling defect in the left posterolateral wall of the bladder. The filling defect is of soft tissue attenuation.
 Transitional cell carcinoma (TCC): TCC must be included in the differential diagnosis of this lesion, but some features mitigate against this as the most likely diagnosis. Large intravesical transitional cell tumors are frequently of the papillary variety. They have a stippled surface and are unlikely to appear as smooth, as in this case. The location will dictate whether a large lesion of this size will obstruct the ureter. In this case, no ureteral obstruction was present.
Transitional cell carcinoma (TCC): TCC must be included in the differential diagnosis of this lesion, but some features mitigate against this as the most likely diagnosis. Large intravesical transitional cell tumors are frequently of the papillary variety. They have a stippled surface and are unlikely to appear as smooth, as in this case. The location will dictate whether a large lesion of this size will obstruct the ureter. In this case, no ureteral obstruction was present.
 Hematoma or fungus ball: There are a multitude of nonfixed filling defects that can occur within the bladder. It is useful in cases where hematomas or fungus balls are being considered to image the patient using ultrasound to document that these are not fixed to the bladder wall. While ultrasound was not performed in this patient, it is essential to document whether filling defects within the bladder are likely to be mobile or fixed.
Hematoma or fungus ball: There are a multitude of nonfixed filling defects that can occur within the bladder. It is useful in cases where hematomas or fungus balls are being considered to image the patient using ultrasound to document that these are not fixed to the bladder wall. While ultrasound was not performed in this patient, it is essential to document whether filling defects within the bladder are likely to be mobile or fixed.
 Bladder calculus: Almost all urinary calculi are hyper-attenuating on CT (> +100 Hounsfield units [HU]). This filling defect measured soft tissue attenuation.
Bladder calculus: Almost all urinary calculi are hyper-attenuating on CT (> +100 Hounsfield units [HU]). This filling defect measured soft tissue attenuation.
 Cystitis: Bullous cystitis can appear as a bladder wall lesion. Cystitis glandularis is a proliferative lesion in which glandular elements of the bladder mucosa occur in the submucosa. Many patients have infections and associated cystitis cystica. These masses are typically villous. Submucosal fluid-filled cysts describe cystitis cystica, which can cause filling defects within the bladder. Chronic infection is postulated as the chief etiologic factor.
Cystitis: Bullous cystitis can appear as a bladder wall lesion. Cystitis glandularis is a proliferative lesion in which glandular elements of the bladder mucosa occur in the submucosa. Many patients have infections and associated cystitis cystica. These masses are typically villous. Submucosal fluid-filled cysts describe cystitis cystica, which can cause filling defects within the bladder. Chronic infection is postulated as the chief etiologic factor.
 Bladder leiomyoma: Smooth muscle tumors of the bladder wall may have this appearance and should be considered in the differential diagnosis of a smooth bladder wall filling defect.
Bladder leiomyoma: Smooth muscle tumors of the bladder wall may have this appearance and should be considered in the differential diagnosis of a smooth bladder wall filling defect.
DIAGNOSIS
Leiomyoma of the bladder
KEY FACTS
Clinical
 Leiomyomas may occur in any site in the genitourinary tract. These lesions occur in all age groups and affect both sexes equally.
Leiomyomas may occur in any site in the genitourinary tract. These lesions occur in all age groups and affect both sexes equally.
 Lesions may be endovesical (63%), intramural (7%), or extravesical (30%).
Lesions may be endovesical (63%), intramural (7%), or extravesical (30%).
 The cause of these tumors is unknown.
The cause of these tumors is unknown.
 The tumor is usually asymptomatic and may be detected incidentally on physical examination or cystoscopy.
The tumor is usually asymptomatic and may be detected incidentally on physical examination or cystoscopy.
 The endovesical form may present with irritative urinary symptoms, gross hematuria, or obstructive symptoms.
The endovesical form may present with irritative urinary symptoms, gross hematuria, or obstructive symptoms.
 Small endovesical lesions can be managed with transurethral resection and fulguration. Larger endovesical, intramural, or extravesical tumors are best treated with segmental resection.
Small endovesical lesions can be managed with transurethral resection and fulguration. Larger endovesical, intramural, or extravesical tumors are best treated with segmental resection.
 The prognosis of this tumor is excellent. No malignant degeneration has been reported.
The prognosis of this tumor is excellent. No malignant degeneration has been reported.
Radiologic
 Intravenous urography or cystography usually reveals a smooth filling defect within the bladder.
Intravenous urography or cystography usually reveals a smooth filling defect within the bladder.
 CT is useful to determine consistency (attenuation), size, location, and possible adjacent organ involvement.
CT is useful to determine consistency (attenuation), size, location, and possible adjacent organ involvement.
 The endovesical form can be sessile or pedunculated on cystoscopy and is usually covered with normal bladder mucosa.
The endovesical form can be sessile or pedunculated on cystoscopy and is usually covered with normal bladder mucosa.
SUGGESTED READING
Fasih N, Prasad Shanbhogue AK, Macdonald DB, et al. Leiomyomas beyond the uterus: unusual locations, rare manifestations. Radiographics 2008;28:1931–1948.
Illescas FF, Baker ME, Weinerth JL. Bladder leiomyoma: Advantages of sonography over computed tomography. Urol Radiol 1986;8:216–218.
Knoll LD, Segura JW, Scheithauer BW. Leiomyoma of the bladder. J Urol 1986;136:906–908.
Wong-You-Cheong JJ, Woodward PJ, Manning MA, Sesterhenn IA. From the Archives of the AFIP: neoplasms of the urinary bladder: Radiologic-pathologic correlation. Radiographics 2006;26:553–580.
M. GENA
FREDERICK
HISTORY
A 63-year-old woman has microscopic hematuria.
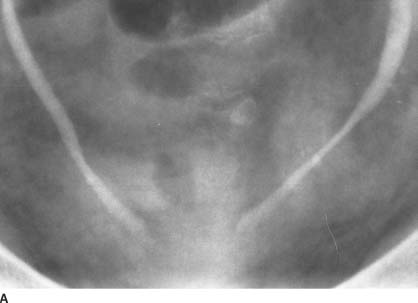
 FIGURE 4-6A Anteroposterior supine 5-minute film of the urinary bladder from an intravenous urogram. There is bilateral columnation of both ureters to the level of the ureterovesical junction, with a rounded distal configuration.
FIGURE 4-6A Anteroposterior supine 5-minute film of the urinary bladder from an intravenous urogram. There is bilateral columnation of both ureters to the level of the ureterovesical junction, with a rounded distal configuration.
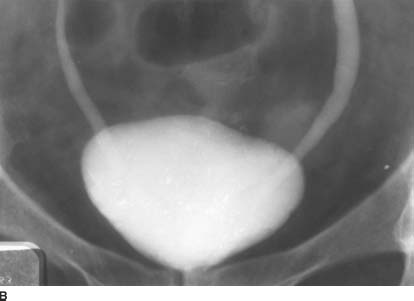
 FIGURE 4-6B Anterosuperior supine 15-minute film of the urinary bladder from an intravenous urogram. Within the bladder, there is a “cobra head” appearance bilaterally. Note the lucent outline of the bulbous ureteral termination.
FIGURE 4-6B Anterosuperior supine 15-minute film of the urinary bladder from an intravenous urogram. Within the bladder, there is a “cobra head” appearance bilaterally. Note the lucent outline of the bulbous ureteral termination.
 Pseudoureterocele: This appearance is caused by a TCC of the bladder or a stone obstructing the ureter. It is unlikely because of the lack of a filling defect or mass, as well as the bilaterality of the defects. Other less common causes of the “pseudoureterocele” appearance include cervical carcinoma invading the ureterovesical orifice, radiation cystitis, or edema of the ureterovesical junction from recent stone passage. However, these are unlikely in this case because the former are identified by asymmetry of the distal lumen and irregularity of the wall and generally do not have intravesicular protrusion. However, they are capable of distending the distal ureter and thus mimicking an orthotopic ureterocele.
Pseudoureterocele: This appearance is caused by a TCC of the bladder or a stone obstructing the ureter. It is unlikely because of the lack of a filling defect or mass, as well as the bilaterality of the defects. Other less common causes of the “pseudoureterocele” appearance include cervical carcinoma invading the ureterovesical orifice, radiation cystitis, or edema of the ureterovesical junction from recent stone passage. However, these are unlikely in this case because the former are identified by asymmetry of the distal lumen and irregularity of the wall and generally do not have intravesicular protrusion. However, they are capable of distending the distal ureter and thus mimicking an orthotopic ureterocele.
 Bilateral simple ureteroceles: This is the most likely diagnosis given the lack of a bladder mass or irregularity, the intravesicular protrusion, the absence of upper tract dilatation, and the bilaterality.
Bilateral simple ureteroceles: This is the most likely diagnosis given the lack of a bladder mass or irregularity, the intravesicular protrusion, the absence of upper tract dilatation, and the bilaterality.
DIAGNOSIS
Bilateral simple ureteroceles
KEY FACTS
Clinical
 An orthotopic ureterocele forms in a ureter with a normal insertion into the trigone, as opposed to an ectopic ureterocele.
An orthotopic ureterocele forms in a ureter with a normal insertion into the trigone, as opposed to an ectopic ureterocele.
 Orthotopic ureteroceles usually occur in single collecting systems, as opposed to ectopic ureteroceles, which occur in duplicated collecting systems.
Orthotopic ureteroceles usually occur in single collecting systems, as opposed to ectopic ureteroceles, which occur in duplicated collecting systems.
 Orthotopic ureteroceles are usually unilateral, asymptomatic, and incidental. However, a calculus may lodge or form in the ureterocele.
Orthotopic ureteroceles are usually unilateral, asymptomatic, and incidental. However, a calculus may lodge or form in the ureterocele.
 A ureterocele is a congenital deformity.
A ureterocele is a congenital deformity.
 A ureterocele consists of a prolapse of the distal ureter into the bladder with associated dilation of the distal ureter.
A ureterocele consists of a prolapse of the distal ureter into the bladder with associated dilation of the distal ureter.
 The wall of the ureterocele is composed of a thin layer of muscle between the outer surface of the bladder uroepithelium and the inner surface of the ureteral uroepithelium.
The wall of the ureterocele is composed of a thin layer of muscle between the outer surface of the bladder uroepithelium and the inner surface of the ureteral uroepithelium.
Radiologic
 The typical radiographic appearance is the so-called cobra head deformity, which is formed by the projection of the minimally dilated distal ureter into the lumen of the bladder, with opacified urine surrounding the ureterocele.
The typical radiographic appearance is the so-called cobra head deformity, which is formed by the projection of the minimally dilated distal ureter into the lumen of the bladder, with opacified urine surrounding the ureterocele.
 The thin line of radiolucency represents the wall of the ureterocele.
The thin line of radiolucency represents the wall of the ureterocele.
SUGGESTED READING
Berrocal T, López-Pereira P, Arjonilla A, Gutiérrez J. Anomalies of the distal ureter, bladder, and urethra in children: embryologic, radiologic, and pathologic features. Radiographics 2002;22:1139–1164.
Davidson AJ, Hartman DS. Radiology of the Kidney and Urinary Tract (2nd ed). Philadelphia: Saunders, 1994;520-523.
Mitty HA, Schapira HE. Ureterocele and pseudoureterocele: cobra versus cancer. J Urol 1977;117:557–561.
M. GENA
FREDERICK
AND
DANIELE MARIN
HISTORY
A 54-year-old man presents with abdominal pain.
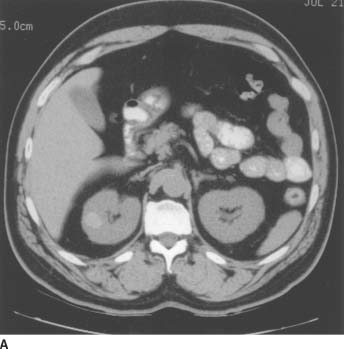
 FIGURE 4-7A Noncontrast CT of the abdomen demonstrates a small, well-defined lesion in the lateral portion of the right kidney with increased attenuation (+59 Hounsfield units [HU]) compared to the adjacent renal tissue.
FIGURE 4-7A Noncontrast CT of the abdomen demonstrates a small, well-defined lesion in the lateral portion of the right kidney with increased attenuation (+59 Hounsfield units [HU]) compared to the adjacent renal tissue.
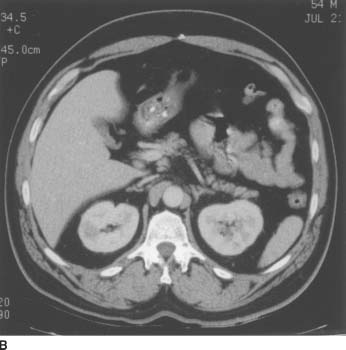
 FIGURE 4-7B On corresponding contrast-enhanced CT image, the lesion demonstrates lack of contrast enhancement (attenuation increase compared to the noncontrast scan = +1 HU).
FIGURE 4-7B On corresponding contrast-enhanced CT image, the lesion demonstrates lack of contrast enhancement (attenuation increase compared to the noncontrast scan = +1 HU).
 Hemorrhagic renal cell carcinoma (RCC): This diagnosis is unlikely because the entire lesion is of uniform increased attenuation on the noncontrast study.
Hemorrhagic renal cell carcinoma (RCC): This diagnosis is unlikely because the entire lesion is of uniform increased attenuation on the noncontrast study.
 Hemorrhagic angiomyolipoma: This diagnosis is unlikely due to the absence of macroscopic fat on the noncontrast CT.
Hemorrhagic angiomyolipoma: This diagnosis is unlikely due to the absence of macroscopic fat on the noncontrast CT.
 Hemorrhagic renal cyst: This is the most likely diagnosis due to the small size, uniform appearance, and lack of enhancement of this renal lesion.
Hemorrhagic renal cyst: This is the most likely diagnosis due to the small size, uniform appearance, and lack of enhancement of this renal lesion.
DIAGNOSIS
Hemorrhagic renal cyst
KEY FACTS
Clinical
 Renal cysts account for approximately 60% of all renal masses.
Renal cysts account for approximately 60% of all renal masses.
 Renal cysts increase in frequency with age (approximately 50% of cases occur past the age of 50).
Renal cysts increase in frequency with age (approximately 50% of cases occur past the age of 50).
 Most renal cysts are asymptomatic, whether hemorrhagic or not.
Most renal cysts are asymptomatic, whether hemorrhagic or not.
 Hemorrhagic cysts are frequently seen in patients with autosomal dominant polycystic kidney disease and acquired renal cystic disease.
Hemorrhagic cysts are frequently seen in patients with autosomal dominant polycystic kidney disease and acquired renal cystic disease.
Radiologic
 Noncontrast CT is necessary to evaluate the attenuation of the lesion before contrast material administration.
Noncontrast CT is necessary to evaluate the attenuation of the lesion before contrast material administration.
 Cysts that are “hyperdense” exhibit attenuation values between +50 and +80 HU. The high attenuation is due to a high content of protein, blood breakdown products, or iodine. To be considered a benign hyperdense cyst, the lesion must be sharply marginated, homogeneous, and nonenhancing (< 10 HU increase postcontrast).
Cysts that are “hyperdense” exhibit attenuation values between +50 and +80 HU. The high attenuation is due to a high content of protein, blood breakdown products, or iodine. To be considered a benign hyperdense cyst, the lesion must be sharply marginated, homogeneous, and nonenhancing (< 10 HU increase postcontrast).
 Because of the thickness of the wall and the internal structure of the lesion, these cysts cannot be evaluated reliably with ultrasound, and only 50% of hyper-attenuating lesions demonstrate typical sonographic cyst criteria. CT is necessary to assess or rule out lesion enhancement.
Because of the thickness of the wall and the internal structure of the lesion, these cysts cannot be evaluated reliably with ultrasound, and only 50% of hyper-attenuating lesions demonstrate typical sonographic cyst criteria. CT is necessary to assess or rule out lesion enhancement.
SUGGESTED READING
Israel GM, Bosniak MA. How I do it: evaluating renal masses. Radiology 2005;236:441–450.
Israel GM, Bosniak MA. Pitfalls in renal mass evaluation and how to avoid them. Radiographics 2008;28:1325–1338.
Bosniak MA. The small (≤ 3.0 cm) renal parenchymal tumor: detection, diagnosis, and controversies. Radiology 1991;179:307–317.
RICHARD A.
LEDER
AND
DANIELE MARIN
HISTORY
A 33-year-old man has a history of urinary tract infections.
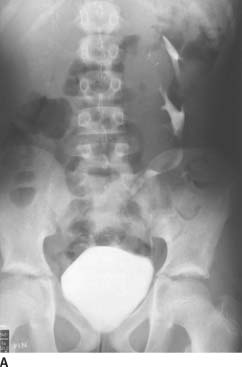
 FIGURE 4-8A Anteroposterior film of the kidneys, ureters, and bladder from an intravenous urogram shows two collecting systems on the left side of the abdomen. On this film, only the distal left ureter is seen
FIGURE 4-8A Anteroposterior film of the kidneys, ureters, and bladder from an intravenous urogram shows two collecting systems on the left side of the abdomen. On this film, only the distal left ureter is seen
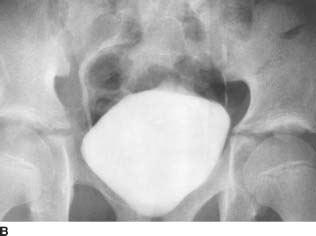
 FIGURE 4-8B Anteroposterior coned view of the pelvis from the intravenous urogram shows normal insertion into the bladder trigone of both the left and right ureters.
FIGURE 4-8B Anteroposterior coned view of the pelvis from the intravenous urogram shows normal insertion into the bladder trigone of both the left and right ureters.
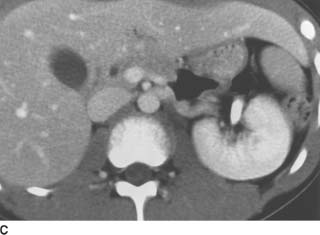
 FIGURE 4-8C Contrast-enhanced CT of the midabdomen shows enhancement of the left kidney, with absence of renal tissue in the right renal fossa.
FIGURE 4-8C Contrast-enhanced CT of the midabdomen shows enhancement of the left kidney, with absence of renal tissue in the right renal fossa.
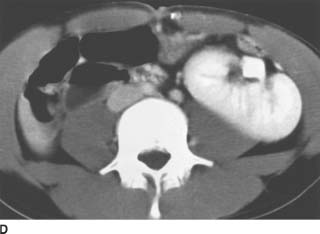
 FIGURE 4-8D Contrast-enhanced CT of the lower abdomen. The right kidney is present in the left lower quadrant.
FIGURE 4-8D Contrast-enhanced CT of the lower abdomen. The right kidney is present in the left lower quadrant.
 Retroperitoneal mass with displacement of the kidney: This diagnosis is unlikely due to the absence of an obvious abdominal mass.
Retroperitoneal mass with displacement of the kidney: This diagnosis is unlikely due to the absence of an obvious abdominal mass.
 Renal duplication with agenesis of the contralateral kidney: Although renal duplication may cause enlargement of the kidney and could account for the presence of two left ureters, this diagnosis can be ruled out with a careful inspection of the course of both ureters, which demonstrates crossing of one ureter into the opposite side of the pelvis and normal insertion in the bladder trigone.
Renal duplication with agenesis of the contralateral kidney: Although renal duplication may cause enlargement of the kidney and could account for the presence of two left ureters, this diagnosis can be ruled out with a careful inspection of the course of both ureters, which demonstrates crossing of one ureter into the opposite side of the pelvis and normal insertion in the bladder trigone.
 Crossed renal ectopia: This is the most likely diagnosis, given the position of the kidneys and the insertion of the ureters.
Crossed renal ectopia: This is the most likely diagnosis, given the position of the kidneys and the insertion of the ureters.
DIAGNOSIS
Crossed renal ectopia
KEY FACTS
Clinical
 There are four types of crossed renal ectopia: (i) crossed renal ectopia with fusion, (ii) crossed renal ectopia without fusion, (iii) solitary crossed renal ectopia, where monolateral renal agenesis is associated with ureteral insertion on the opposite side of the solitary kidney, and (iv) bilateral crossed renal ectopia, where both kidneys are crossed to the opposite side of the abdomen with their ureters inserting into the contralateral ureterovesicle junction.
There are four types of crossed renal ectopia: (i) crossed renal ectopia with fusion, (ii) crossed renal ectopia without fusion, (iii) solitary crossed renal ectopia, where monolateral renal agenesis is associated with ureteral insertion on the opposite side of the solitary kidney, and (iv) bilateral crossed renal ectopia, where both kidneys are crossed to the opposite side of the abdomen with their ureters inserting into the contralateral ureterovesicle junction.
 The most common varieties are fused and unfused ectopia; crossed fused ectopia occurs in 85% to 90% of cases.
The most common varieties are fused and unfused ectopia; crossed fused ectopia occurs in 85% to 90% of cases.
 Crossed renal ectopia is seen more commonly in males than females.
Crossed renal ectopia is seen more commonly in males than females.
 The most common scenario is the left kidney crossing to the right side of the abdomen.
The most common scenario is the left kidney crossing to the right side of the abdomen.
 There are associated urinary tract abnormalities, including obstruction, stones, infection, vesicoureteral reflux, primary megaureter, hypospadius, cryptorchi-dism, urethral valves, and multicystic dysplastic kidney.
There are associated urinary tract abnormalities, including obstruction, stones, infection, vesicoureteral reflux, primary megaureter, hypospadius, cryptorchi-dism, urethral valves, and multicystic dysplastic kidney.
 There are associated abnormalities of other organ systems, including skeletal anomalies, unilateral ovarian and fallopian tube agenesis, and cardiac and gastrointestinal anomalies.
There are associated abnormalities of other organ systems, including skeletal anomalies, unilateral ovarian and fallopian tube agenesis, and cardiac and gastrointestinal anomalies.
 Theories of occurrence include faulty development of the ureteral bud with crossing of the midline to contact the contralateral metanephric blastema, obstruction of renal ascent by blood vessels, and local environmental factors involving surrounding tissues and organs.
Theories of occurrence include faulty development of the ureteral bud with crossing of the midline to contact the contralateral metanephric blastema, obstruction of renal ascent by blood vessels, and local environmental factors involving surrounding tissues and organs.
Radiologic
 The most common form of crossed renal ectopia is a crossed fused ectopia. Radiographically, this can be diagnosed on either ultrasound, CT, or intravenous urography when renal tissue lies on the opposite side of the abdomen from its ureteral insertion, and renal tissue from the crossed kidney fuses with the kidney native to that side of the abdomen. Helical CT, particularly using coronal reformation, or MRI may be useful in distinguishing fused from unfused ectopia.
The most common form of crossed renal ectopia is a crossed fused ectopia. Radiographically, this can be diagnosed on either ultrasound, CT, or intravenous urography when renal tissue lies on the opposite side of the abdomen from its ureteral insertion, and renal tissue from the crossed kidney fuses with the kidney native to that side of the abdomen. Helical CT, particularly using coronal reformation, or MRI may be useful in distinguishing fused from unfused ectopia.
 CT is also useful for excluding secondary causes of renal displacement, such as large retroperitoneal masses.
CT is also useful for excluding secondary causes of renal displacement, such as large retroperitoneal masses.
 Patients with this abnormality are usually asymptomatic, although they may present with a palpable abdominal mass or a history of repeated urinary tract infections.
Patients with this abnormality are usually asymptomatic, although they may present with a palpable abdominal mass or a history of repeated urinary tract infections.
SUGGESTED READING
Gay SB, Armistead JP, Weber ME, Williamson BR. Left infrarenal region: anatomic variants, pathologic conditions, and diagnostic pitfalls. Radiographics 1991;11:549–570.
Silva JM, Jafri SZH, Cacciarelli AA, et al. Abnormalities of the kidney: embryogenesis and radiologic appearance. Appl Radiol 1995;24:19–24.
RICHARD A.
LEDER
AND
DANIELE MARIN
HISTORY
A 60-year-old man presents with a palpable right-sided abdominal mass, flank pain, and hematuria.
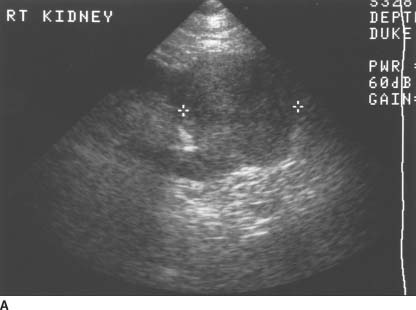
 FIGURE 4-9A Transabdominal ultrasound of the right kidney in the longitudinal plane shows a solid, hyperechoic, partially exophytic mass at the lower pole.
FIGURE 4-9A Transabdominal ultrasound of the right kidney in the longitudinal plane shows a solid, hyperechoic, partially exophytic mass at the lower pole.
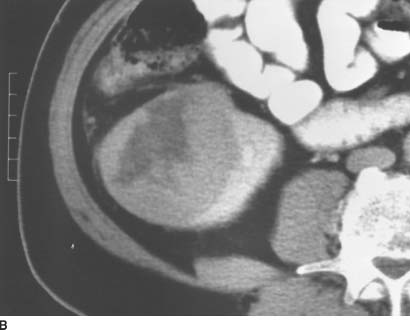
 FIGURE 4-9B Contrast-enhanced CT shows a large, solid mass in the right kidney. Within the central and lateral portion of the mass, there is a broad area of decreased attenuation, corresponding to areas of cystic tumor necrosis.
FIGURE 4-9B Contrast-enhanced CT shows a large, solid mass in the right kidney. Within the central and lateral portion of the mass, there is a broad area of decreased attenuation, corresponding to areas of cystic tumor necrosis.
 Renal cell carcinoma (RCC): The imaging features in this case reveal the presence of a solid, enhancing right renal mass with features that are consistent with RCC. The broad area of low attenuation within the mass could represent internal hemorrhage or necrosis.
Renal cell carcinoma (RCC): The imaging features in this case reveal the presence of a solid, enhancing right renal mass with features that are consistent with RCC. The broad area of low attenuation within the mass could represent internal hemorrhage or necrosis.
 Oncocytoma: There are no imaging features that confidently allow for the diagnosis of a benign oncocytoma. However, this diagnosis belongs in the differential diagnosis of a solitary renal mass in a patient who has no evidence of metastatic disease (no retroperitoneal lymphadenopathy and no osseous, hepatic, or pulmonary parenchymal metastases).
Oncocytoma: There are no imaging features that confidently allow for the diagnosis of a benign oncocytoma. However, this diagnosis belongs in the differential diagnosis of a solitary renal mass in a patient who has no evidence of metastatic disease (no retroperitoneal lymphadenopathy and no osseous, hepatic, or pulmonary parenchymal metastases).
 Renal metastasis: Metastatic disease to the kidney is uncommon and would be only included in the differential diagnosis of patients with a known primary malignancy, most commonly lung, breast, or colon cancer. No such history existed in this patient.
Renal metastasis: Metastatic disease to the kidney is uncommon and would be only included in the differential diagnosis of patients with a known primary malignancy, most commonly lung, breast, or colon cancer. No such history existed in this patient.
 Angiomyolipoma: The diagnosis of angiomyolipoma is made when fat is detected within a renal mass. It is possible that given sufficient hemorrhage within an angiomyolipoma, no fat may be detected. No fat was detected within this mass, and there was no evidence of subcapsular or perinephric hemorrhage.
Angiomyolipoma: The diagnosis of angiomyolipoma is made when fat is detected within a renal mass. It is possible that given sufficient hemorrhage within an angiomyolipoma, no fat may be detected. No fat was detected within this mass, and there was no evidence of subcapsular or perinephric hemorrhage.
 Lymphoma: This patient has no history of non-Hodg-kin’s lymphoma. Furthermore, no retroperitoneal lymphadenopathy is present, although lymphomatous masses may exist within the kidneys in the absence of lymphadenopathy.
Lymphoma: This patient has no history of non-Hodg-kin’s lymphoma. Furthermore, no retroperitoneal lymphadenopathy is present, although lymphomatous masses may exist within the kidneys in the absence of lymphadenopathy.
DIAGNOSIS
Renal oncocytoma
KEY FACTS
Clinical
 An “oncocyte” is a transformed epithelial cell with an enlarged, homogeneous, dense cytoplasm filled with acidophilic granules.
An “oncocyte” is a transformed epithelial cell with an enlarged, homogeneous, dense cytoplasm filled with acidophilic granules.
 Microscopically, a renal oncocytoma is characterized by eosinophilic epithelial cells with protuberant mitochondria within the cytoplasm.
Microscopically, a renal oncocytoma is characterized by eosinophilic epithelial cells with protuberant mitochondria within the cytoplasm.
 A renal oncocytoma has a distal tubular or collecting duct origin.
A renal oncocytoma has a distal tubular or collecting duct origin.
 On gross examination, lesions are well circumscribed, often encapsulated, without necrosis or hemorrhage. A central stellate scar may be present.
On gross examination, lesions are well circumscribed, often encapsulated, without necrosis or hemorrhage. A central stellate scar may be present.
 The right kidney is affected as often as the left kidney. Cases of bilateral synchronous tumors have been reported.
The right kidney is affected as often as the left kidney. Cases of bilateral synchronous tumors have been reported.
 The tumor size ranges from 0.1 to 26.0 cm.
The tumor size ranges from 0.1 to 26.0 cm.
 The age at diagnosis ranges from 26 to 94 years.
The age at diagnosis ranges from 26 to 94 years.
 There is a 1.63 to 1.0 male-to-female ratio.
There is a 1.63 to 1.0 male-to-female ratio.
 Less than one-third of patients will present with the classic triad of renal cell tumors, including flank pain, hematuria, with a palpable abdominal mass.
Less than one-third of patients will present with the classic triad of renal cell tumors, including flank pain, hematuria, with a palpable abdominal mass.
 Rarely, renal oncocytomas may demonstrate a malignant growth pattern with distant metastatic disease.
Rarely, renal oncocytomas may demonstrate a malignant growth pattern with distant metastatic disease.
Radiologic
 Ultrasound shows a homogeneous, isoechoic to hyperechoic, well-marginated mass. These are indistinguishable from RCC.
Ultrasound shows a homogeneous, isoechoic to hyperechoic, well-marginated mass. These are indistinguishable from RCC.
 Although rarely performed, angiography may demonstrate a “spoke-wheel” appearance due to the characteristic vascular architecture of the tumor. Other common but less specific angiographic findings include a dense parenchymal blush and no contrast media puddling, arteriovenous shunting, or renal vein invasion as commonly seen in RCC.
Although rarely performed, angiography may demonstrate a “spoke-wheel” appearance due to the characteristic vascular architecture of the tumor. Other common but less specific angiographic findings include a dense parenchymal blush and no contrast media puddling, arteriovenous shunting, or renal vein invasion as commonly seen in RCC.
 On CT, oncocytomas demonstrate well-defined and smooth margins, with or without a central stellate scar. Lesions demonstrate intense and homogeneous enhancement after dynamic administration of contrast material.
On CT, oncocytomas demonstrate well-defined and smooth margins, with or without a central stellate scar. Lesions demonstrate intense and homogeneous enhancement after dynamic administration of contrast material.
 Only 67% of large oncocytomas (> 3 cm) and 82% smaller tumors (< 3 cm) demonstrate typical imaging findings at CT (i.e., homogeneous attenuation throughout the tumor, a central, sharply marginated stellate area of low attenuation). In the remaining cases (33% and 18%, respectively), CT in unable to differentiate oncocytomas from RCC.
Only 67% of large oncocytomas (> 3 cm) and 82% smaller tumors (< 3 cm) demonstrate typical imaging findings at CT (i.e., homogeneous attenuation throughout the tumor, a central, sharply marginated stellate area of low attenuation). In the remaining cases (33% and 18%, respectively), CT in unable to differentiate oncocytomas from RCC.
 On MRI, oncocytomas show homogenous, low signal intensity on T1-weighted images and high signal intensity on T2-weighted images. The presence of a capsule or a central stellate scar and the absence of either internal hemorrhage or necrosis also favor this diagnosis.
On MRI, oncocytomas show homogenous, low signal intensity on T1-weighted images and high signal intensity on T2-weighted images. The presence of a capsule or a central stellate scar and the absence of either internal hemorrhage or necrosis also favor this diagnosis.
SUGGESTED READING
Davidson AJ, Hayes WS, Hartman DS, et al. Renal oncocytoma and carcinoma: failure of differentiation with CT. Radiology 1993;186:693–696.
Hélénon O, Merran S, Paraf F, et al. Unusual fat-containing tumors of the kidney: a diagnostic dilemma. Radiographics 1997;17:129–144.
Velasquez G, Glass TA, D’Souza VJ, Formanek AG. Multiple oncocytomas and renal carcinoma. AJR Am J Roentgenol 1984;142:123–124.
RICHARD A.
LEDER
AND
DANIELE MARIN
HISTORY
A 48-year-old man was referred for CT after seeing his ophthalmologist.
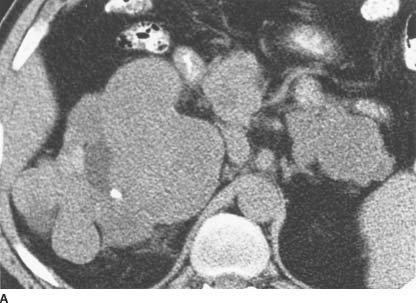
 FIGURE 4-10A Noncontrast CT of the abdomen shows enlargement of both the head and tail of the pancreas, although it is difficult to determine whether there are solid or cystic lesions present. There is marked deformity of the right kidney, with a hydrocalyx containing a small calculus in the upper pole, exophytic renal masses, and a solid mass medially.
FIGURE 4-10A Noncontrast CT of the abdomen shows enlargement of both the head and tail of the pancreas, although it is difficult to determine whether there are solid or cystic lesions present. There is marked deformity of the right kidney, with a hydrocalyx containing a small calculus in the upper pole, exophytic renal masses, and a solid mass medially.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 CHAPTER EDITOR
CHAPTER EDITOR