Fig. 8.1
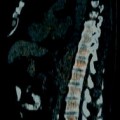
Computed tomography (CT)–guided renal biopsy (nontargeted). A noncontrast axial CT image demonstrates a biopsy needle within the right kidney. Note the tangential course of the needle with respect to the renal cortex
Ablation of Renal Tumors
Although surgical resection remains the standard of care for localized renal cell carcinoma, ablative techniques such as cryoablation and radiofrequency ablation are rapidly becoming viable alternatives for treatment of small renal lesions in patients who may not be able to tolerate surgical resection. Ablative techniques can be applied either through an open, laparoscopic, or percutaneous approach.
Cryoablation uses a rapid freeze–thaw cycle to both mechanically disrupt cell membranes and draw out intracellular fluid resulting in alterations in intracellular pH. Radiofrequency ablation uses frictional energy to heat surrounding tissues, resulting in cell death. A recent meta-analysis suggests that, based on short-term oncologic outcomes, ablative treatments are reasonable options in the treatment of small renal masses [11]. Levinson et al. [12] published early long-term outcomes following patients treated with radiofrequency ablation to a mean of 61.6 months, citing an overall recurrence-free survival of 90.3 %. Such results lead these authors to suggest that radiofrequency ablation can play a role in the management of small renal lesions in patients who are high-risk surgical candidates or have a limited life expectancy [12].
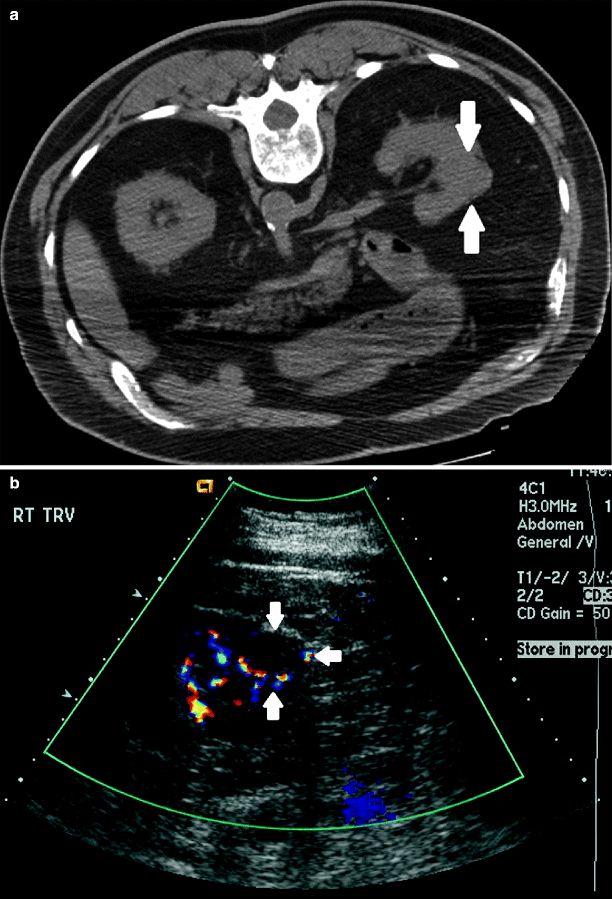
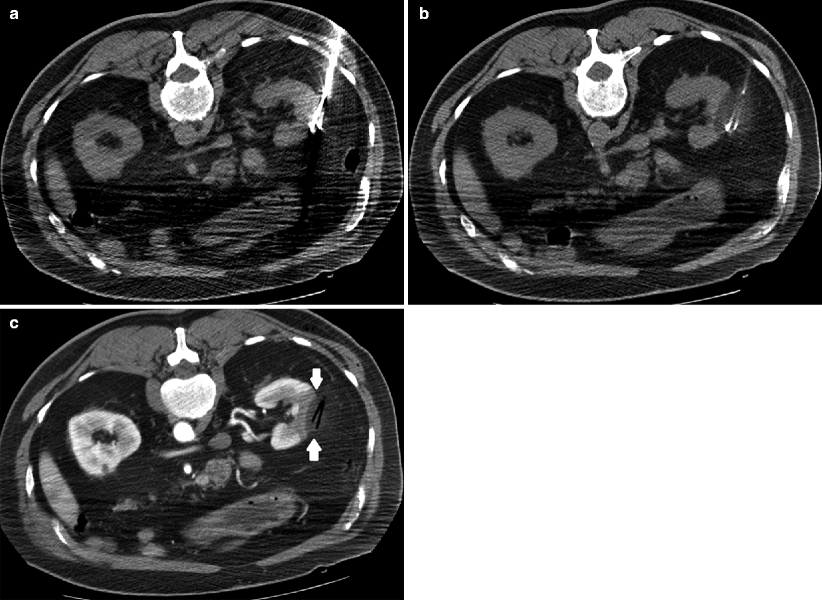
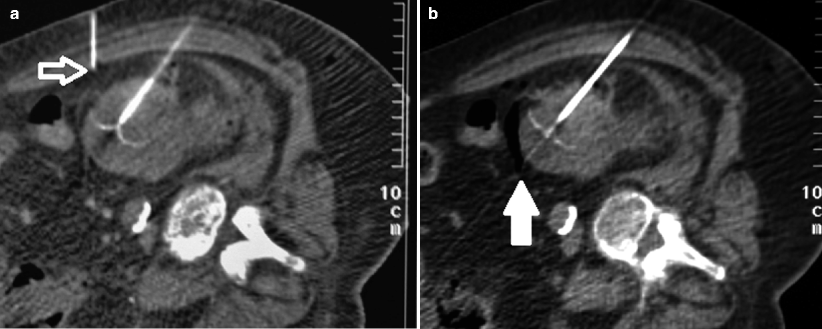

Fig. 8.2
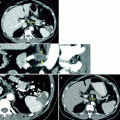
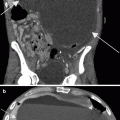
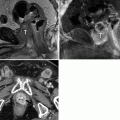
Renal mass. (a) Noncontrast images demonstrate contour irregularity of the right kidney at the level of the known renal mass (arrows), better appreciated on a preprocedural contrast-enhanced CT (not shown). (b) Due to the inconspicuous nature of this lesion, initial targeting was done by ultrasound. Ultrasound through the same region clearly demonstrates a mass with peripheral vascularity (arrows). Targeting needles or the ablation probe can be placed in the renal mass under ultrasound guidance while the patient is on the CT gantry

Fig. 8.3
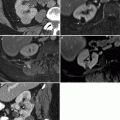
Cryoablation. Axial images demonstrating the cryoprobes in place (a) with low density surrounding the probes (b) representing ice ball formation. In solid organs such as the kidney, ice ball formation can be well visualized and allows for better approximation of ablation margins. (c) Post-contrast CT after ablation demonstrates a low-density defect (arrows) representing the ablated area with air density in the probe tract

Fig. 8.4
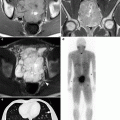
Advanced separation techniques. On occasion, vital structures such as hollow viscera (bowel) or vasculature may be too close to the target lesion for that lesion to be safely ablated. (a, b) An ablation probe is fully deployed in the target renal lesion; however, the edge of the tumor is too close to the adjacent bowel. Air or fluid can be used as an insulator to the ablation energy. A small needle (open arrow in a) was in the space between the target lesion and bowel. Air was subsequently injected to separate the organs (solid arrow in b)
Role of Arterial Embolization
Embolization of Renal Angiomyolipomas
Renal angiomyolipoma (AML) is the most common benign renal neoplasm. Treatment of an AML is generally reserved for patients presenting with hemorrhage or pain, or with the goal to preempt possible spontaneous bleeding from a large lesion. Transcatheter arterial embolization is an important adjunct before complete or partial nephrectomy, and in many cases can serve as the primary therapy [13]. Although there is little argument for the emergent treatment of a hemorrhagic AML, controversy exists regarding treatment of minimally symptomatic and asymptomatic AML. Most centers use a size cutoff of 4 cm for elective treatment. The vast majority of tumors larger than 4 cm are symptomatic and more than half are associated with a risk of spontaneous hemorrhage [14, 15].
Transcatheter arterial embolization can be performed with various agents: absolute ethanol, with or without ethiodized oil, polyvinyl alcohol particles, absorbable gelatin powder, spherical particles, coils, or a combination. There remains no consensus as to the embolic material of choice. Technical success rates have been reported in the range of 90–100 %. Recurrence rates (same site), especially in those patients with associated tuberous sclerosis complex, can be up to 30 % [16]. For patients treated for symptoms secondary to mass effect or for primary size reduction, long-term mean size reduction can be seen ranging from 43 to 70.2 % [17, 18].
Angiographically, the vessels of an AML are tortuous, as is the case for tumor vasculature. Active contrast extravasation in the setting of acute hemorrhage may not always be seen on angiography as the surrounding hematoma may partially obscure the bleeding. Authors have described the use of a “light bulb” sign to direct embolization. Similar to a light bulb, a bleeding nidus/aneurysm resembles a filament of an incandescent and the surrounding hematoma resembles the bulb [19].
Postembolization follow-up imaging generally demonstrates cessation of bleeding (if treatment was for hemorrhage) and decreased tumor size. Since the fatty component of the AML is relatively insensitive to embolization, there is much variability in size reduction, and thus size itself cannot be used to determine treatment success [18]. There is currently no consensus on the follow-up of patients after embolization for AML. Some authors suggest that short-term imaging is predictive of long-term outcome [17]. Kothary et al. [16] have suggested lifelong follow-up for patients with associated tuberous sclerosis complex, secondary to high tumor-recurrence rates.
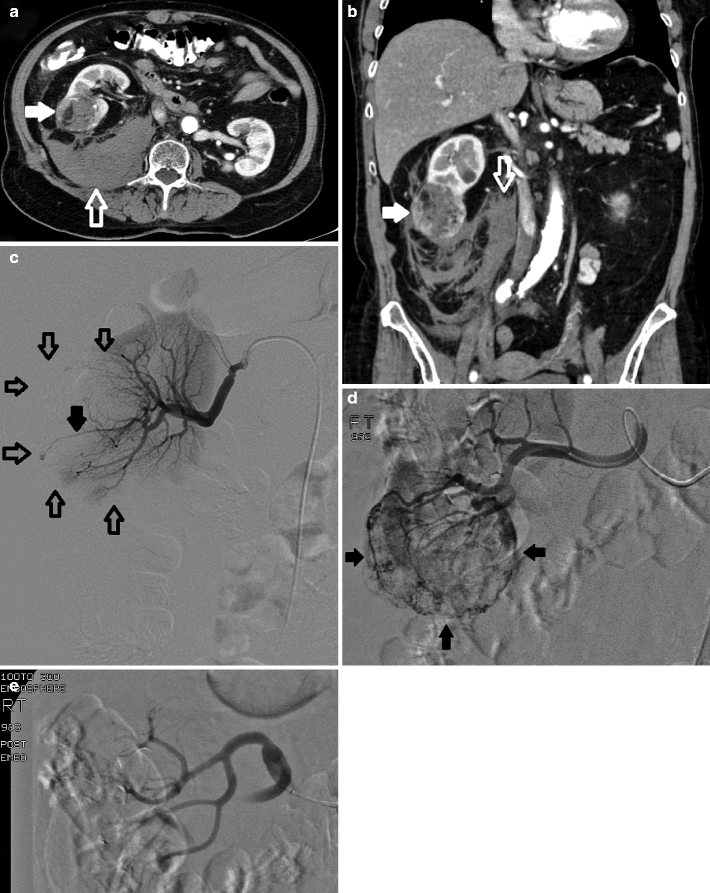

Fig. 8.5
Ruptured AML. Post-contrast axial (a) and reformatted coronal CT images (b) demonstrate an exophytic mass (solid arrows) arising from the right kidney containing macroscopic fat. There is a large amount of perirenal and retroperitoneal hemorrhage (open arrows), consistent with a ruptured AML. (c) Selective renal arteriogram demonstrating an irregular renal parenchymal contour secondary to surrounding hematoma. Additionally, a single tortuous vessel (solid arrow) with a nidus and surrounding lack of parenchymal blush (open arrows) is seen, compatible with the region of hemorrhage, the “light bulb sign.” (d) Superselective renal arteriogram with microcatheter demonstrates filling of the tumor (solid arrows) with associated tortuous tumor vasculature. (e) Postembolization arteriogram demonstrates stasis of contrast in the renal artery with no filling of the tumor
Preoperative Embolization for Renal Neoplasms
A relatively controversial indication for renal artery embolization is that of preoperative embolization of tumor prior to partial or complete nephrectomy [20, 21]. Proponents of this procedure cite decreased operative time and blood loss, and ease of tissue dissection created by edema in dissection planes after embolization [22]. One retrospective analysis suggests decreased perioperative blood loss in patients undergoing preoperative embolization of tumors larger than 10 cm [23]. However, prospective randomized trials are lacking and thus are required to identify the role of embolization in the preoperative setting.
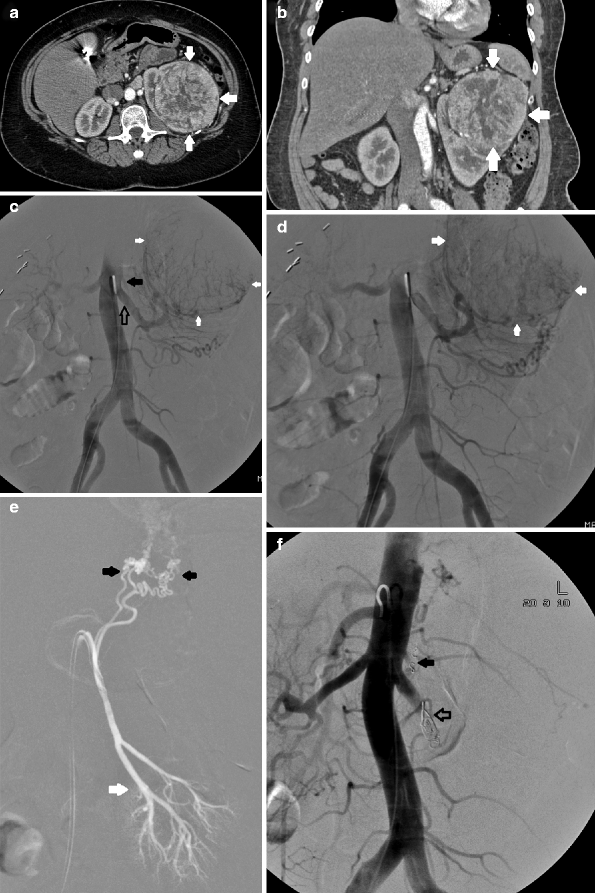

Fig. 8.6
Renal mass. Axial (a) and reformatted coronal (b) CT images demonstrate a large heterogeneously enhancing renal mass (arrows) replacing a large portion of left renal parenchyma. Due to its size, preoperative arterial embolization was performed with the goal of decreasing intraoperative blood loss. (c) Aortogram demonstrates multiple tumor vessels supplying the mass (solid white arrows) as noted on CT. Of note, an accessory renal artery (solid black arrow) in addition to the main renal artery (open black arrow) is seen. (d) Aortogram mid-embolization demonstrates contrast within the mass (arrows). (e) Accessory renal artery. Selective arteriogram demonstrates that the accessory renal artery feeds both tumor (white arrows) and normal residual renal parenchyma (black arrow). Because this patient would be undergoing total nephrectomy, coil embolization after particle embolization was performed. (f) Completion angiogram demonstrates no filling distal to the coils within the left main (open arrow) or accessory renal artery (solid arrow)
Embolization of Pseudoaneurysm After Partial Nephrectomy
A recent 5-year outcomes analysis suggests that laparoscopic and open partial nephrectomy are comparable in overall and cancer-specific survival in the treatment of small tumors [24]. Hemorrhage after both open and laparoscopic partial nephrectomy is a known complication, with one study citing a 9.5 % hemorrhage rate after laparoscopic partial nephrectomy [25,26]. Rarely do patients require therapy beyond conservative management; however, a small subset of patients may develop pseudoaneurysms, resulting in gross hematuria and decreased hematocrit, some of which are potentially life threatening. Catheter-directed embolization of these pseudoaneurysms can be performed with excellent technical and clinical success [27]. Pseudoaneurysms may also be seen rarely after renal biopsy, placement of percutaneous nephrostomies or tumor ablations, and are treated in a similar fashion.
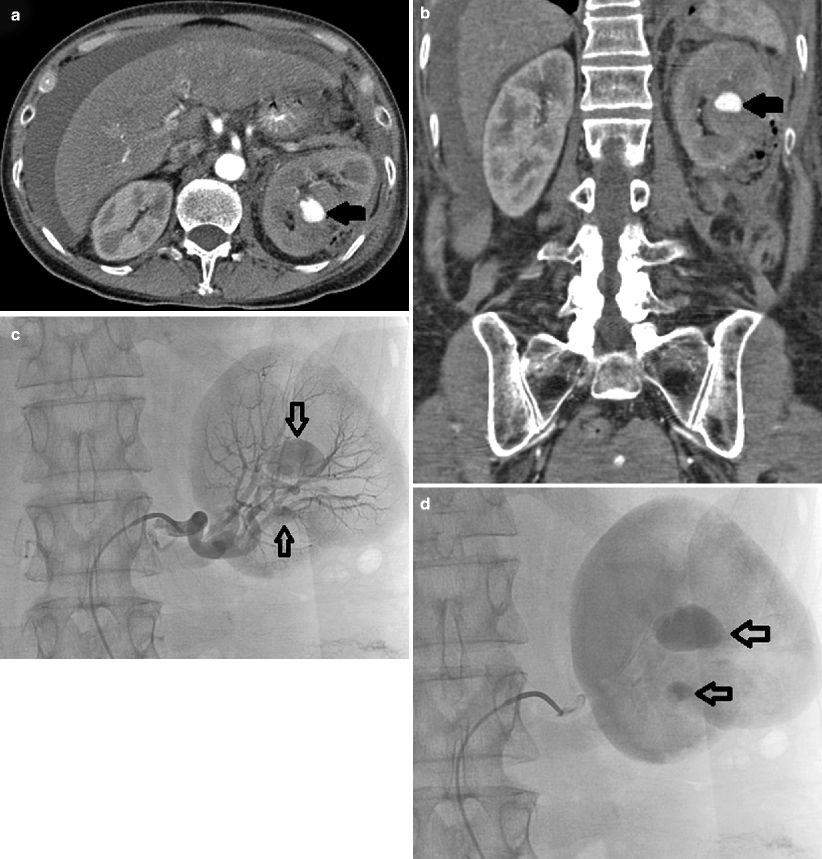
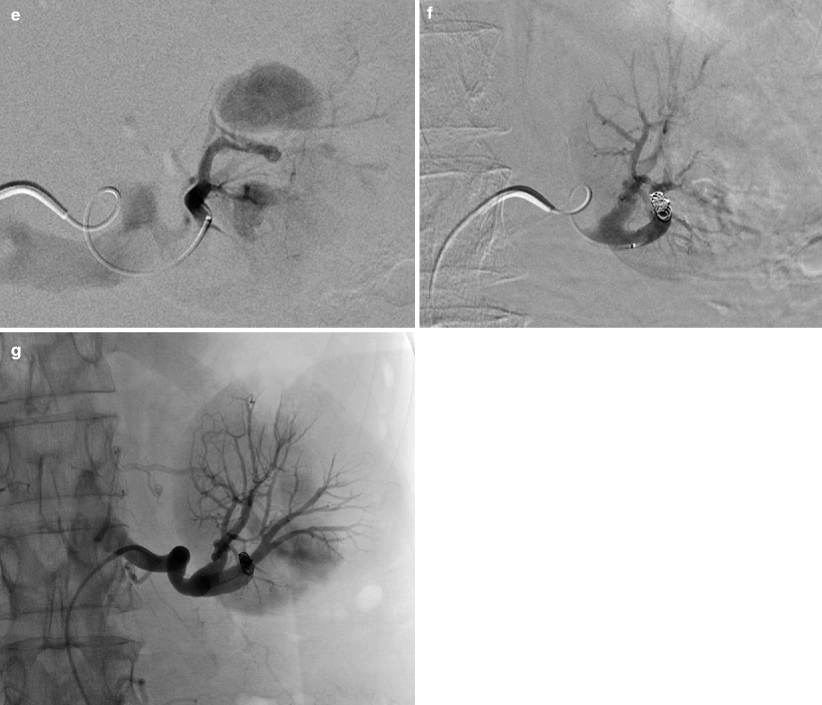


Fig. 8.7
CT. Post-contrast axial (a) and reformatted coronal CT images (b) demonstrate a large pseudoaneurysm (arrows) abutting the left partial nephrectomy bed. This patient has had persistent hematuria and decreased hematocrit for several days postoperatively. One of the most common causes of postnephrectomy-delayed hemorrhage is pseudoaneurysm [28]. (c) A selective left renal arteriogram demonstrates two pseudoaneurysms (open arrows) abutting the surgical bed (early arterial), which become more apparent as focal pools of contrast on parenchymal phase (d). (e) Superselective injection. Superselective angiogram demonstrates that both pseudoaneurysms arise from the same subsegmental artery. Careful diagnostic arteriogram technique should always be followed as it will often guide therapy and, in this case, allow for minimal sacrifice of normal residual renal parenchyma. (f, g) Postembolization. After coil embolization, repeat arteriogram, both subselective (f) and from the main renal artery (g), demonstrates complete exclusion of the pseudoaneurysms
Radiologic Therapies in Ureteral Obstruction
Percutaneous access and tube placement within an obstructed renal collecting system was first described in 1955 by Goodwin et al. [29]. Since then, indications for nephrostomy tube placement have evolved to include obstructive uropathy, urinary diversion, treatment of nephrolithiasis, and treatment of obstructive urosepsis. Furthermore, technological advances have yielded multiple different types of percutaneously placed catheters, such as the nephrostomy tube, nephroureterostomy tube, and the ureteral stent (JJ stent).
In the oncologic patient, the choice of these different catheters is based on the etiology of the obstruction, disease prognosis, and patient comfort. For instance, a completely internalized ureteral stent, would be the most comfortable as patients would not be required to have a catheter with or without an attached bag extending from the flank. However, a completely internalized catheter would ideally need to be placed and replaced in a retrograde fashion and would initially require visualization and access to the ureteral orifice. Thus, patients with bladder tumors that distort underlying anatomy may not be ideal candidates. Generally, if the urinary bladder is normal, placement of internal stents is suggested as the first choice for patients. For those immediately involved in placement and management of these catheters, it is important to be aware of the advantages and disadvantages of each, which is beyond the scope of this chapter. Dyer et al. [30], however, provide an excellent review of these catheters.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree