, Albert J. Chang1, Alexander R. Gottschalk1 and Mack RoachIII2
(1)
Department of Radiation Oncology, University of California San Francisco, 1600 Divisadero Street, Suite H1031, San Francisco, CA, USA
(2)
Department of Radiation Oncology and Urology, University of California San Francisco, 1600 Divisadero Street, Suite H1031, San Francisco, CA, USA
Pearls
Prostate adenocarcinoma is considered to have low α[alpha]/β[beta] ratio of approximately 1.5–3, making it conducive to hypofractionated treatment.
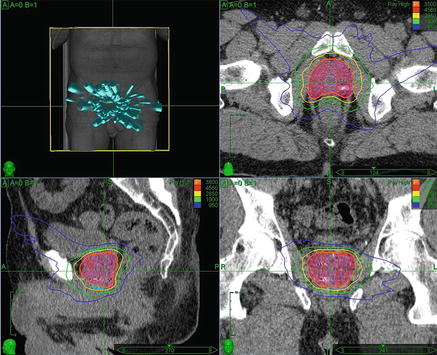
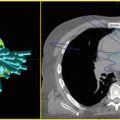
Fig. 9.1
(b) Axial, (c) sagittal, and (d) coronal views of an example prostate SBRT plan. Patient with low risk, cT1c, Gleason 6 prostate cancer with PSA of 3.8 treated with SBRT monotherapy to 38 Gy in 4 fractions. Target volume is shown (shaded red), along with urethral avoidance structure (blue). Prescription isodose line is shown in orange, and 120 % of the prescription dose is shown in red. A typical plan uses many (>100) non-coplanar , non-isocentric beams, as shown in (a)
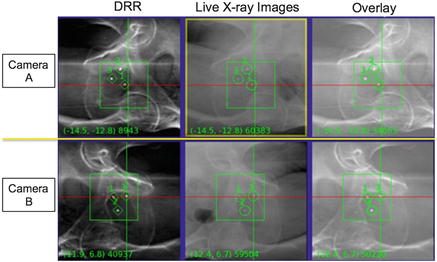
Fig. 9.2
Intrafraction fiducial tracking used for CK prostate treatments. The three gold fiducial markers placed in the prostate are visualized on orthogonal DRRs generated from the planning CT (left), and on the live kV X-ray images (middle). During treatment delivery, beams are automatically re-targeted based on the registration of fiducials on the two images (right) to account for intrafraction motion (within specific translational and rotational bounds)
Renal cell carcinoma α[alpha]/β[beta] ratio relatively low (3–6).
Few studies on SBRT for bladder, renal, or other GU sites.
Most prostate studies done using CyberKnife , but standard linear accelerator -based SBRT acceptable.
Prostate SBRT more cost-effective and convenient for patients than IMRT (Sher et al 2012).
No randomized trials assessing efficacy of SBRT compared to standard treatments.
Treatment Indications
Disease site | Presentation | Recommended treatment |
|---|---|---|
Prostate | Low risk | Active surveillance, RP, or definitive radiation (IMRT with IGRT, brachytherapy monotherapy, or SBRT monotherapy) |
Intermediate risk | RP or definitive radiation (IMRT with prostate-specific boost using external beam radiation, brachytherapy, or SBRT boost), with short-term ADT (4 months). Consider HDR or SBRT monotherapy in select favorable patients | |
High risk | RP or definitive radiation (IMRT with prostate-specific boost using external beam radiation, brachytherapy, or SBRT boost), with long-term ADT (2–3 years) | |
Residual disease after RT | RP, salvage HDR, or SBRT | |
Bladder | Muscle invasive disease (T2-T4), bladder preservation candidate | +/− neoadj chemo → radical cystectomy or concurrent chemo-RT with IMRT to bladder and pelvis; consider SBRT boost to tumor bed (investigational) |
Renal cell carcinoma | Unilateral disease, medically operable | Nephrectomy +/− post-op RT |
Unilateral disease, medically inoperable | SBRT to primary lesion | |
Bilateral or recurrent contralateral disease | SBRT to lesion in unresected kidney |
Work-Up
Prostate
Work-up performed per standard-of-care depending on risk group.
H&P, focusing on urinary symptoms, erectile function, bone pain , and DRE .
Labs: PSA , Testosterone, and LFTs for intermediate- and high-risk patients anticipating androgen deprivation.
TRUS-guided biopsy with >8 cores, with prostate volume measurement.
Bone scan or NaF PET/CT for any of the following (per NCCN 2014):
PSA > 20.
T2 and PSA > 10.
GS > 7.
T3 or T4.
Symptoms.
Pelvic CT or MRI for T3, T4 or a probability of lymph node involvement >10 % (per NCCN 2014).
Prostate MRI for treatment planning.
Bladder
H&P, labs: CBC , BUN , CR, Alk Phos, UA, Urine cytology .
Cystoscopy with EUA .
Upper urinary tract imaging (IVP , CT urography, renal U/S, ureteroscopy, or MRI urogram ).
If muscle invasive, Chest XR or CT, Abdominal/pelvic CT.
TURBT with random bladder biopsies , including prostatic urethra if trigone involved.
Renal Cell Carcinoma
H&P (hematuria, flank pain , flank mass most common presenting symptoms).
Labs: CBC , LFTs, BUN , Cr, LDH , UA.
CT abdomen, or MRI abdomen if concern for IVC involvement.
CXR or CT Chest.
Bone scan , brain MRI only if clinically indicated.
Radiosurgical Technique
Simulation and Field Design
Prostate
TRUS-guided placement of at least 3 gold seed marker s (2 at base, 1 at apex) at least 1 week prior to simulation .
Simulation: Enema day of simulation , full bladder, supine with alpha cradle .
Fuse prostate MRI with simulation CT.
Consider enema 2–3 h prior to each treatment (per RTOG 0938).
Image Guidance
CyberKnife:
Real-time fiducial tracking using orthogonal kV x-ray fluoroscopy for intrafraction motion (preferred).
Linac:
Daily image guidance using fiducials via EPID , conebeam CT or helical tomo CT imaging .
If treatment time >7 min, repeat IGRT procedure during treatment at least every 7 min.
Repeat IGRT procedure at end of treatment to document stability during treatment.
Treatment planning:
GTV : any lesion visible on MRI and/or based on biopsy information.
CTV : prostate +/− proximal Seminal Vesicle s on CT/MRI fusion .
PTV : CTV + 3–5 mm expansion, sparing rectum .
Contour urethra as avoidance structure .
Bladder
Placement of 3–4 gold seed marker s delineating tumor bed during TUBRT.
Simulation: Supine with alpha cradle . Full vs. empty bladder controversial: less bowel toxicity with full bladder; more setup reproducibility with empty bladder. At UCSF, we simulate with full bladder.
Image Guidance
CyberKnife: Real-time fiducial tracking for intrafraction motion (preferred).
Linac: Daily image guidance with fiducials, with EPID , CBCT , or helical tomo CT.
Treatment planning:
GTV : Macroscopic tumor visible on CT/MRI /Cystoscopy .
CTVboost: GTV + 0.5 cm + tumor bed as delineated by gold seed marker s .
PTV = CTV + 1.5 cm.
Renal Cell Carcinoma
Simulation: Supine with alpha cradle , arms above head.
Obtain 4D-CT to define ITV .
Image guidance:CyberKnife: Real-time fiducial tracking
Daily conebeam CT.
Field design
GTV : Tumor visible on planning CT.
ITV : Integrated tumor volume based on movement on 4D-CT.
PTV : ITV + 3–5 mm.
Contour surrounding normal kidney as avoidance structure .
Dose Prescriptions
Prostate SBRT monotherapy
Acceptable regimens: 7.25 Gy × 5 (most common), 9.5 Gy × 4.
QOD treatment given increased toxicity with daily fractionation.
Consider simultaneous integrated boost to GTV if dominant lesion visible on MRI .
Prostate SBRT boost
Acceptable regimens: 9.5 Gy × 2, 7 Gy × 3. At UCSF we use 9.5 Gy × 2.
Prostate post-RT salvage
6 Gy × 5.
If focal recurrence demonstrated on biopsy and/or MRI , consider partial volume treatment to dominant intraprostatic lesion.
Bladder SBRT boost
3.5 Gy × 5 (equivalent to 20 Gy boost in 2 Gy fractions assuming α[alpha]/β[beta] = 10).
Renal cell carcinoma
10 Gy × 4 (or 10 Gy × 3 if large).
Dose Limitations
Structure | Fractions | Constraints | Source |
|---|---|---|---|
Rectum | 4 5 | V75% < 2 cc Dmax < 105 % | UCSF (Jabbari et al 2012) RTOG 0938 |
Urethra | 4 5 | V120% < 10 % Dmax < 107 % | UCSF (Jabbari et al 2012) RTOG 0938 |
Bladder | 4 5 | V75% < 3 cc Dmax < 105 % | UCSF (Jabbari et al 2012) RTOG 0938 |
Penile bulb | 5 | V54% < 3 cc Dmax < 100 % | RTOG 0938 |
Femoral heads | 5 | V54% < 10 cc Dmax < 30 Gy
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|