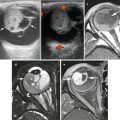
Fig. 11.1
Mediastinal teratoma. (a) Axial and (b) coronal contrast-enhanced chest CT in a 2-year-old boy shows a mixed solid, cystic, fatty and calcific lesion occupying most of the left chest. Narrowing and stretching of the left pulmonary artery is evident on the axial image. It is difficult to exclude pleural or chest wall invasion from the coronal images. (c) Coronal contrast-enhanced CT in another patient again showing a thoracic mass lesion with solid, calcific and fatty components

Fig. 11.2
Ovarian germ cell tumour. An ovarian GCT which appeared as a largely cystic hypovascular mass in an 8-year-old girl with only small solid components inferiorly
After sonographic assessment, further detailed evaluation with cross-sectional imaging is ideally performed with magnetic resonance imaging (MRI), which will typically show cysts with varying contents, from a simple fluid signal to complex proteinaceous fluids. Conventional T1-weighted (T1W) MRI will also easily demonstrate the fatty components of GCTs as high signal foci, paralleling the signal from subcutaneous fat (the fatty components, in terms of imaging appearances, will mirror fat elsewhere on all sequences). MRI with gadolinium optimally demonstrates the solid components and septa of GCTs [4]. The relationship, and infiltrative nature when present, of the mass to other viscera will further become apparent on MRI. Similarly, MRI is the best method to assess for intraspinal tumour extension, such as can occur with sacrococcygeal teratomas for example. When MRI is unavailable then CT will also show lesion extent to good effect, albeit with an inherent irradiation burden. CT is the most sensitive modality for detecting foci of calcification, a common occurrence, within these tumours and also readily demonstrates the fatty components of these masses [4]. Malignant tumours in general have more solid components than benign lesions, but with the exception of detecting metastases with some malignant disease, imaging generally cannot predict whether a lesion is benign or malignant.
Associations of Paediatric Germ Cell Tumours
An association between pediatric GCTs and haematological malignancies has been reported, (including acute myeloid leukaemia, histiocytoses and mastocytosis), usually in association with mediastinal malignant germ cell tumours in young adult males but occasionally also with ovarian germ cell tumours. In such cases the haematological malignancy may arise concurrently or several months after the initial GCT [5]. At least a proportion of such cases, particularly those developing many months following the GCT, may be chemotherapy related [6].
For most pediatric GCTs there are no strong epidemio- logical associations with environmental factors [7] and no strong genetic associations reported, although both mediasinal and intracranial GCTs are more frequent in patients with Klinefelter syndrome [8]. In contrast to adult testicular GCTs which frequently demonstrate isochromosome 12p, there are no characteristic cytogenetic abnormalities in childhood cases, although chromosome 1p deletions leading to loss of heterozygosity (LOH) are reported more frequently in childhood malignant GCTs [9] and array CGH approaches have demonstrated that whilst infant teratomas show no consistent abnormality, in older children changes similar to adult cases, such as chromosome 12p gains, may be identified [10]. However, whilst molecular diagnostic testing does not currently play a major role in pediatric GCT diagnosis or prognostication, it is likely that new generation technologies may identify additional molecular relationships, previously undetectable using cytogenetic approaches [11].
General Histological Features of Germ Cell Tumours
GCTs represent neoplasms derived from primordial germ cells, which may theoretically differentiate into a wide range of recognisable mature tissue types in addition to forming specific tumour types based on proliferation of immature and abnormal germ cells. Therefore, all GCTs can, in principle, be histologically composed of either a pure population demonstrating one specific histological phenotype, or may represent a mixture of multiple different GCT components. Frankly malignant elements generally represent the least differentiated GCT elements and initial management is usually based on the predominant malignant element present [2, 3].
Rarely, secondary malignancies may arise within pre-existing GCTs, in which the malignant element represents secondary transformation of an underlying teratomatous element, rather than a primary malignant GCT element. This has been reported to include both a range of carcinomas and sarcomas, but when this occurs in childhood the malignant elements present are usually those recapitulating either primitive neuroectodermal tumour or rhabdomyosarcoma [12, 13].
From a practical clinical perspective, due to the potential marked heterogeneity in histological tissue composition within a GCT, of which a malignant element such as yolk sac tumour, which is overall by far the commonest malignant germ cell tumour component in childhood, may only represent a minority of the entire tumour mass. As a consequence caution must always be exercised when interpreting the results of a small image-guided biopsy (Fig. 11.3). There has been there a marked recent move in many pediatric centres towards primary diagnosis of most pediatric tumours using image-guided needle core biopsies rather than open biopsy or resection, especially in the UK and Europe. Such an approach can usually provide an adequate amount of material for diagnosis in the vast majority of cases but due to the issues of limited sampling, malignant elements of a GCT may not be sampled, and the results of such biopsies should be interpreted in light of other clinical features including the results of serum biomarkers such as alpha fetoprotein (AFP) and human chorionic gonadotropin (HCG) [14].
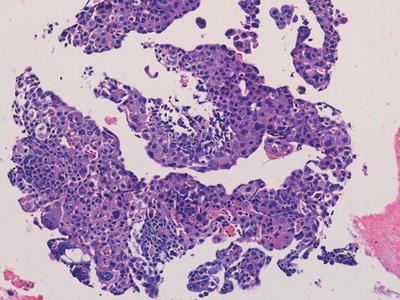

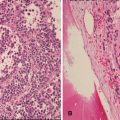
Fig. 11.3
Photomicrograph of material obtained from a thoracic/lung biopsy in a child with a large mass lesion and raised serum human chorionic gonadotropin level demonstrating irregular fragments and sheets of cells recapitulating the morphological features of trophoblast indicating a choriocarcinomatous component of a malignant mixed germ cell tumour. Original magnification 200×
In addition to the wide range of specific histological subtypes which may be present in GCTs, ranging from mature through to very immature and embryonal tissue, within a given histological subtype there may also be further wide variation in specific patterns of histological appearances which are described. Therefore, GCTs can demonstrate a huge variation in their histological appearances both within a given tumour and between cases. Nevertheless, despite this apparent complexity, from a diagnostic perspective in clinical pathology practice, most of these patterns are of no relevance other than allowing correct recognition of the diagnosis. In routine practice, GCT diagnosis does not usually represent a major diagnostic difficulty in specialist laboratories [15].
Specific Histological Types of Germ Cell Tumours
Teratoma
Teratomas represent neoplasms in which there are histological tissue components recapitulating all three embryonic germ cell layers, which form recognisable structures but a histologically disorganised mass lesion. There may be a wide variation in the proportion showing differentiation towards each of the different germ cell layers and in very rare cases in childhood, monodermal teratomas may exist in which only one element appears present, the characteristic example being struma ovarii of the ovary composed of thyroid-type tissue [16].
In general, the most common elements identified in pediatric teratomas regardless of site are neural components followed by cutaneous tissue, fat, fibrous tissue, cartilage and gastrointestinal type epithelium, but with a wide range of other tissue types being described [2, 3]. Due to the large variation in elements present there is usually a mixture of cystic and solid components, corresponding to the general imaging appearances described above. Depending on the site, teratomas may locally infiltrate widely and markedly distort the normal anatomy, this being particularly the case in congenital tumours in which the teratoma is presumed to have grown in utero; in such cases surgery may be technically very difficult due to the unusual anatomy, including atypical vascular anatomy supplying, or running through, the tumour [17, 18].
In the majority of cases, all elements within a teratoma are histologically mature. Most cases of mature teratoma, provided excision is complete, behave in a benign fashion. There are some exceptions to this, however, specifically that the presence of an apparently mature teratoma within the testis of young adult may be associated with malignant behaviour [19] (Figs. 11.4, 11.5, 11.6, and 11.7).
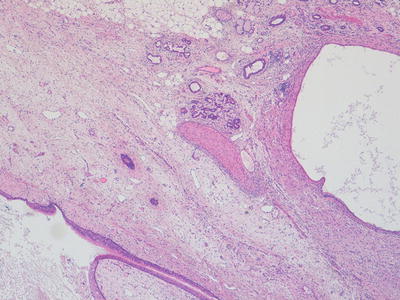
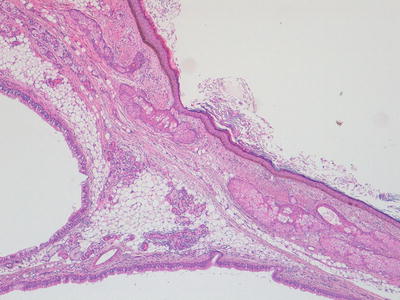
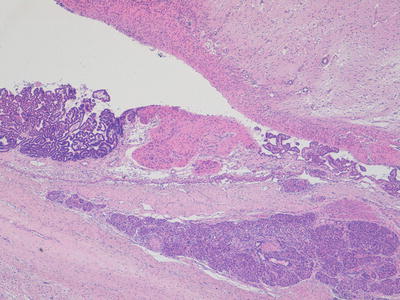
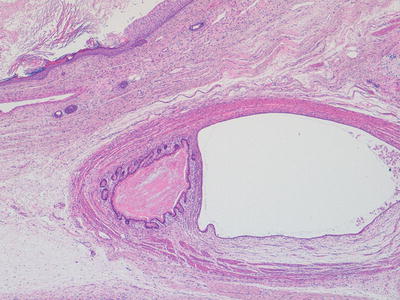

Fig. 11.4
Photomicrograph of a mature teratoma demonstrating a variety of disorganised but mature elements. There are no immature areas. Original magnification 40×

Fig. 11.5
Photomicrograph of a mature teratoma demonstrating a variety of disorganised but mature elements. There are no immature areas. Original magnification 40×

Fig. 11.6
Photomicrograph of a mature teratoma demonstrating a variety of disorganised but mature elements. There are no immature areas. Original magnification 20×

Fig. 11.7
Photomicrograph of a mature teratoma demonstrating a variety of disorganised but mature elements. There are no immature areas. Original magnification 20×
Immature teratomas represent cases in which, in addition to any other tissues present, there is a component of immature embryonal tissue. In most cases, the majority of the tumour represents mature teratoma but with presence of immature neuroepithelial structures, the frequency and extent of which is related to the histological grading of such cases (Figs. 11.8, 11.9, and 11.10). The clinical significance of immature tissue within a teratoma appears to be related to the age of the patient and anatomical site. For example, immature elements in a congenital sacrococcygeal teratoma appear to have little significance for prognosis whereas immature tissue in the ovary of an adolescent girl is of adverse prognostic significance. The diagnosis of immature teratoma should be reserved for cases in which definite immature neuroepithelial tissue can be identified. The presence of apparently immature mesenchymal components, in isolation, is of uncertain significance, and is not usually a clinically relevant issue in immature teratoma of the ovary, in which immature neuroepithelial elements are almost always additionally present. The main relationship in the literature between histological grading of immature teratomas and clinical behaviour and outcome is provided for ovarian immature teratomas in young adults but such data is not available for teratomas at extragonadal sites in younger children [2, 20].
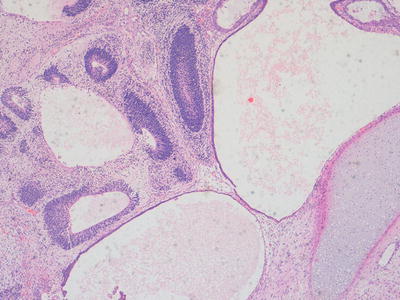
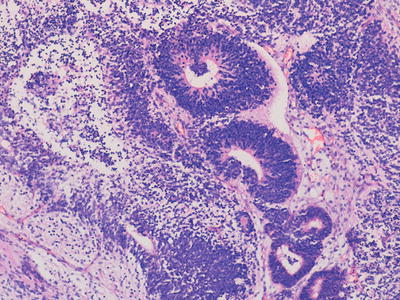
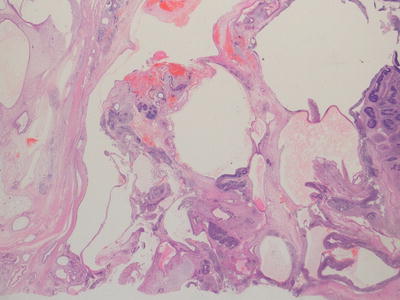

Fig. 11.8
Photomicrograph of a teratoma demonstrating mixed mature and immature elements. There are well differentiated cystic structures and an area of cartilage in the bottom right hand corner whereas in the top left area there are numerous immature neuroepithelial tubules. Original magnification 100×

Fig. 11.9
Photomicrograph of a teratoma demonstrating mixed mature and immature elements. There are well-differentiated cystic structures and numerous immature neuroepithelial tubules. Original magnification ×200

Fig. 11.10
Photomicrograph of a teratoma demonstrating numerous immature neuroepithelial tubules. Original magnification ×20
For the diagnostic pathologist, the most important aspect in the histological diagnosis of a teratomatous GCT is adequate sampling and histological assessment in order to detect any possible areas of malignant elements, almost always represented by yolk sac tumour. Therefore, it is important that the entire tumour is examined and sectioned and any atypical areas are sampled. The clinical management of GCTs in which a small focus of malignant yolk sac tumour is identified depends on the age and stage in terms of completeness of excision. Whilst the serum alpha-fetoprotein is often raised in the presence of the malignant yolk sac elements it should also be recognised that there may be cases in which serum alpha-fetoprotein is raised but despite an extensive examination of the specimen, no yolk sac tumour can be identified. In such cases, it has been suggested that the alphafetoprotein may be derived from other sources such as immature hepatic tissue [2, 3, 21].
Imaging Features
Teratomas manifest as typical GCTs with cystic and solid areas, with lesions in individual areas of the body having a propensity to invade local structures. Some important specific radiological features of intracranial, mediastinal and sacro-coccygeal tumours are described elsewhere in this chapter.
Benign mature teratoma accounts for between 23 and 48 % of prepubertal testicular GCTs. These are usually found in boys under 4 years of age and appear radiologically as complex solid and cystic masses on ultrasound evaluation, often being large relative to the size of the testis. Echogenic foci may be evident as a result of calcification or fat within the lesion [22].
Most ovarian teratomas are diagnosed late in the first decade of life, with a peak age incidence at diagnosis of 10 years, or in early adolescence. The majority are benign. Most patients present as an abdominal mass (Fig. 11.11) or with pain due to torsion of the lesion (Fig. 11.12).
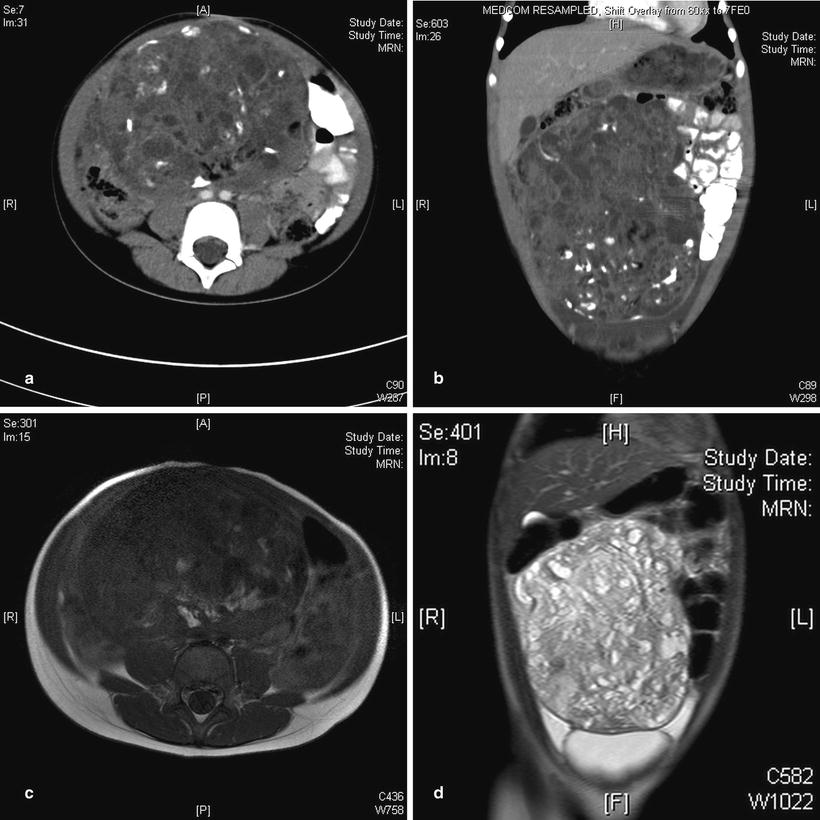
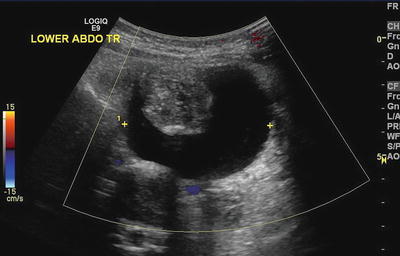

Fig. 11.11
Ovarian teratoma. (a) Axial CT in a 6-year-old girl showing a calcified and cystic mass occupying much of the central abdomen. (b) Coronal CT in the same patient shows the supero-inferior extent of the mass. (c) Conventional T1-weighted image in the same patient shows the fatty components of the mass posteriorly to have hyperintense signal similar to subcutaneous fat. (d) Coronal T2W image again shows the craniocaudal extent of this cystic mass to good effect, with some associated ascites in the lower quadrants being more obvious on this sequence

Fig. 11.12
Ovarian teratoma. A torted ovarian teratoma in an adolescent girl manifested with acute abdominal pain. Ultrasound, with Doppler evaluation, showed a mixed solid and cystic avascular mass
Primary abdominal and retroperitoneal teratomas are extremely rare and represent only 4–6 % of teratomas [23]. Although usually benign, abdominal teratomas in infants are often large and have a particular tendency to infiltrate widely such that describing them as merely retroperitoneal masses is anatomically simplistic. They have a propensity to expand anteriorly and often occupy virtually the entire abdomen such that contact with the anterior abdominal wall is common. Their large size often makes ascribing a definite organ or anatomical compartment of origin difficult. Whilst pancreatic, gastric, mesenteric and gallbladder origins are frequently described for these masses, the location may simply reflect the largest component of a tumour mass originating in the retroperitoneum or root of the mesentery. The typical radiological features of a retroperitoneal teratoma are a large, complex mass, usually with well-circumscribed cystic components, containing fat and areas of calcification [24]. The sonographic appearances of mature retroperitoneal teratoma are usually those of a mixed tumour with cystic and solid components. Calcification and characteristic adipose content or fat-fluid levels are better demonstrated on CT or MRI. Contrast enhancement of an intra-cystic solid component has been suggested as a criterion to distinguish benign from rarer malignant lesions in adults (with a sensitivity and specificity of approximately 80 %) and this may also be applicable to paediatric GCTs [25].
It is noteworthy, that vascular displacement seems to occur in a different pattern in abdominal teratomas to that seen with other infiltrative paediatric tumours such as neuroblastoma. These tumours almost invariably envelop the aorta and vena cava [26]. Mass effect can cause marked distortion of normal anatomical relationships and compression or complete effacement of the IVC. In addition to being compressed or effaced, unusual but marked anterior displacement of the IVC away from the aorta is a surgical risk with these tumours and this should be carefully considered pre-operatively [26] (Fig. 11.13). Furthermore these masses may prove difficult to remove at surgery not only due to vascular encasement but also from being densely adherent to adjacent structures. Current CT angiographic techniques should be adequate to assess the arterial anatomy but the compressed abdominal veins can be very difficult to evaluate even with the best sonographic technique.
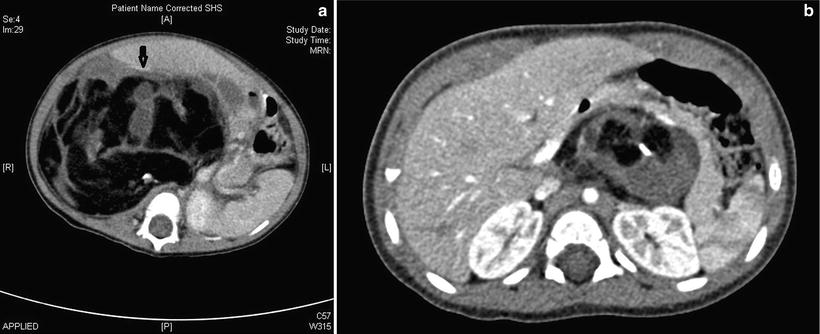

Fig. 11.13
Retroperitoneal teratoma. (a) Axial contrast-enhanced CT in a 6-month-old male infant shows a heterogenous, mainly fatty, retroperitoneal mass in the right flank crossing the midline. The unusual, and surgically hazardous, anterior displacement of the IVC (arrow) is highlighted here. (b) CT in a 5-month-old girl shows a mainly left-sided solid, fatty and calcific retropancreatic mass, anterior to the left kidney
Seminoma/Germinoma
Seminoma and germinoma represent essentially the same neoplasm, depending upon the site and sex of the patient, being composed of sheets of uniform immature germ cells with no significant morphological differentiation towards other tissue types. Germinoma/seminoma occurs predominantly in adults and is generally rare in childhood, although is not uncommon affecting the gonads of teenagers and young adults. In younger children this diagnosis is very uncommon, particularly in its pure form, except in the context of a pineal germ cell tumour [2, 27].
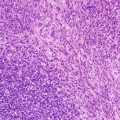
Seminoma/germinomas usually present as solid masses with focal haemorrhage and necrosis and histologically demonstrate a distinctive appearance composed of sheets and nests of bland uniform cells separated by delicate fibrous septa. In most cases there is an associated significant lymphocytic inflammatory infiltrate, which in some cases may initially obscure the underlying seminomatous component. The tumour cells have a characteristic uniform appearance with open vesicular nuclei but some cases can show variable degrees of cytological pleomorphism. In small biopsies, especially with extensive lymphoid tissue present, immunohistochemical staining may be helpful in making the diagnosis [2, 3] (Fig. 11.14).
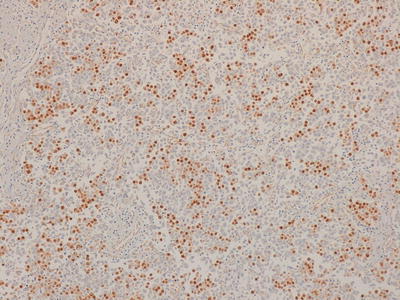
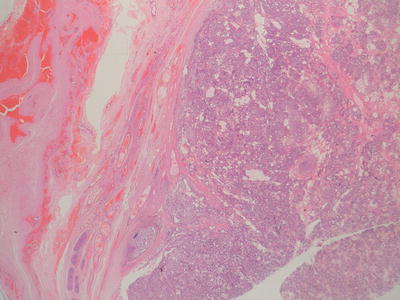

Fig. 11.14
Photomicrograph of a seminoma/germinoma immunostaining for OCT3/4 demonstrating positivity in the majority of cells. Original magnification 100×

Fig. 11.15
Photomicrograph of a testicular malignant yolk sac tumour, which is confined to the testis. Original magnification 20×
Imaging Features
Seminoma, whilst the most common testicular tumour in adults, is very rare in childhood but when it does occur, it is the neoplasm most commonly associated with a cryptorchid testis [22]. Seminomas tend to have homogeneous, soft-tissue attenuation while the more malignant lesions frequently have large necrotic components but there is wide variation in the appearance of all these tumours.
Yolk Sac Tumour (Endodermal Sinus Tumour)
Yolk sac tumour represents by far the most common malignant GCT element in young children. They may occur as pure neoplasms or, more commonly, in association with other GCT elements such as teratoma. In the majority of cases they are associated with raised serum levels of alpha-fetoprotein (AFP).
Macroscopically yolk sac tumours (YST) are usually solid with areas of haemorrhage and necrosis. Micro-scopically, they have been reported as displaying an extremely large variety of histological patterns, which may make diagnosis difficult in some cases on small needle biopsy specimens. Histologically, there is almost always microcyst formation with intervening solid components composed of tumour cells which are relatively uniform small cells with little cytoplasm. The variant architectural features of these cystic and solid components include reticular, pseudopapillary, polyvesicular vitelline and solid patterns, in addition to areas of myxoid change and even pleomorphic sarcoma-like areas. The characteristic histological feature, which is not required for the diagnosis, is the presence of Schiller-Duval bodies, glomeruloid structures appearing to show a central blood vessel covered with tumour cells surrounded by a cyst lined by tumour cells. In most cases, in a resection specimen, the diagnosis is straightforward since a wide range of patterns are exhibited. The only variant which may cause some difficulty in diagnosis is the hepatoid pattern which usually shows solid sheets of cells with eosinophilic cytoplasm recapitulating fetal liver development [2, 3, 28] (Figs. 11.5 and 11.16).
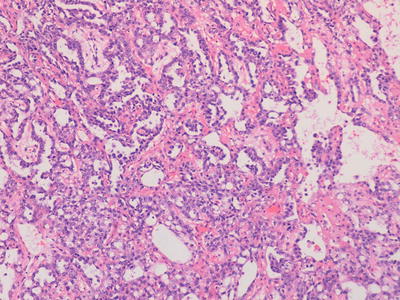

Fig. 11.16
Photomicrograph of a testicular malignant yolk sac tumour, demonstrating characteristic histological patterns. Original magnification 200×
A useful diagnostic feature in general is the presence of hyaline droplets which are present in the vast majority of yolk sac tumours and highlighted by DPAS staining. Yolk sac tumours almost always exhibit strong cytokeratin positivity, patchy expression of alpha fetoprotein, and more recently, glypican-3 immunostaining has been reported as a reliable and sensitive immunomarker for YST [29].
Imaging Features
YSTs appear on imaging similar to other GCTs, but elevated serum levels of AFP suggest the diagnosis. They tend to be more infiltrative than benign GCTs and may arise from the ovary, testis or elsewhere in the abdomen (Fig. 11.17). Testicular yolk sac tumours, which occur mainly in pre-pubertal boys, are confined to the testis (stage 1 disease) in 85 % at presentation [22] (Fig. 11.18). In the upper abdomen yolk sac tumours may mimic a primary hepatic tumour and it can be very difficult to identify the true site of origin of the lesion (Fig. 11.19). Extragonadal yolk sac tumours may occur virtually anywhere in the body, such as in the brain, mediastinum, common bile duct or thyroid gland as examples. Routine chest CT to detect or exclude pulmonary metastases is recommended at diagnosis.
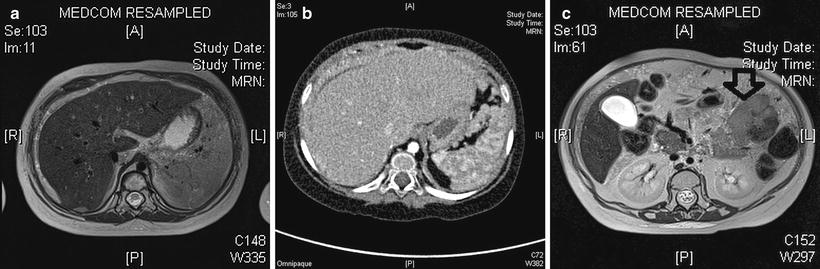
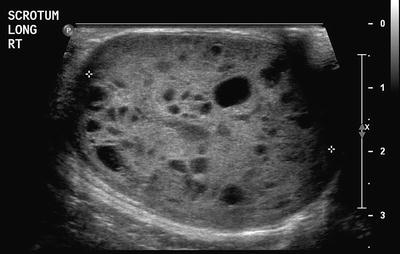
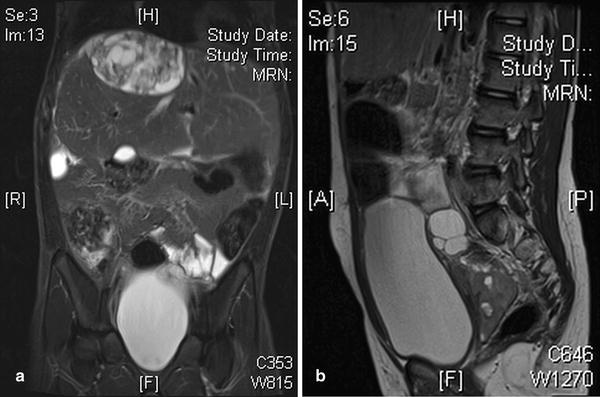

Fig. 11.17
Abdominal yolk sac tumour (YST). (a) Axial T2W image shows tissue similar to subcutaneous fat, albeit with some heterogeneity, peripheral to the liver parenchyma in a 3-year-old girl. (b) Contrast-enhanced CT shows this tissue is not adipose tissue but rather widespread infiltrating tumour tissue from a biopsy proven YST. (c) T2W MRI more inferiorly in the same patient shows a hypointense mass lesion in the left abdomen (arrow) which was also a component of this multifocal YST peritoneal tumour

Fig. 11.18
Testicular yolk sac tumour. This tumour manifested as a cystic, and predominantly solid testicular mass, in a 4-year-old boy

Fig. 11.19
Peritoneal tumour infiltration. This can occur with a mature teratoma or more malignant infiltrating tumours also. (a) An initial mature ovarian teratoma at surgery was found to have numerous small peritoneal deposits with identical pathology. Some of these enlarged over time. Coronal T2W image shows a heterogenous hyperintense mass superior to the right lobe of the liver. This was proven on repeated resection to be entirely mature teratomatous tissue. (b) Sagittal T2W image shows a cystic and solid metastatic deposit in the pouch of Douglas, posterior to a distended bladder, from a yolk sac tumour of the ovary
Embryonal Carcinoma
Pure embryonal carcinoma almost never occurs in childhood and is rare even as a component of a mixed malignant GCT, which is the setting in which it is most commonly encountered. Histologically, embryonal carcinoma displays sheets of large pleomorphic cells with prominent nucleoli in an epithelioid pattern. Immunostaining is helpful for their recognition since they usually express cytokeratins and CD30 [2, 3].
Choriocarcinoma
Choriocarcinoma essentially does not occur in childhood as a primary GCT although it may rarely be a component of a mixed GCT in older children. It should be noted that in other GCTs such as seminoma, germinoma and embryonal carcinoma, scattered syncytiotrophoblast-like giant cells may be present but this does not designate the diagnosis of choriocarcinoma [19].
A hepatic malignancy termed “infantile choriocarcinoma of the liver” has been reported in association with maternal metastatic disease and intraplacental choriocarcinoma, representing metastatic spread from a placental gestational trophoblastic tumour [30] In young teens, at the start of the reproductive period, if pregnancy should occur, there is an increased risk of a hydatidiform molar pregnancy with potential associated risk of persistent gestational trophoblastic disease, however these gestational neoplasms do not represent GCTs. In adolescents extrauterine choriocarcinoma is extremely rare but may occur in the fallopian tube, ovary or elsewhere in the abdomen and pelvis. Ovarian choriocarcinoma accounts for less than 1 % of all ovarian tumours, and the mean age of presentation in children is 13.6 ± 6.9 years [31].
Imaging Features
A hemorrhagic mass or a hemoperitoneum may be seen with an ovarian primary. A large unilateral, hypervascular adnexal mass with multiple cystic cavities and the presence of haemorrhage associated with elevated β–HCG levels and an “empty uterus” are highly suspicious for an ovarian choriocarcinoma [31]. An MRI of the pelvis can confirm the contents of a mass to be hemorrhagic. It may nevertheless be challenging to differentiate choriocarcinoma from ectopic pregnancy in a young woman by imaging findings alone [31]. Distant hematogenous metastases may be present, however, with choriocarcinoma and help point to that diagnosis.
Issues Relating to Germ Cell Tumours at Specific Sites
Gonadal Germ Cell Tumours
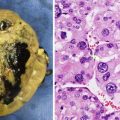
GCTs arising in the gonad have different epidemiological characteristics in boys and girls. In boys there are two distinct peaks of GCT development, corresponding to distinctly different histological entities and underlying pathophysiological basis. GCTs occurring in young children represent either mature or immature teratomas or malignant yolk sac tumour. The majority of these present as asymptomatic mass lesions confined to the testis and do not show an association with intratubular precursor germ cell lesions, almost all of them in young children occurring in otherwise normal testes. Yolk sac tumour in young children does not show the characteristic cytogenetic abnormalities associated with adult testicular GCTs [28] (Figs. 11.20, 11.21, 11.22, 11.23, 11.24, 11.25, and 11.26).
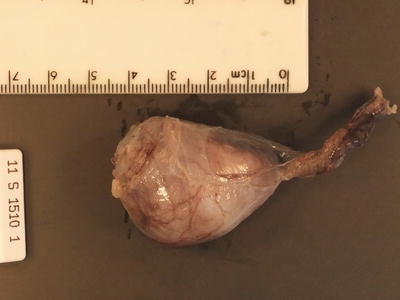
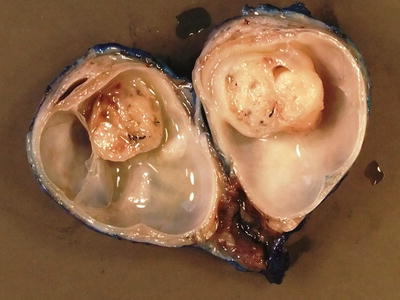

Fig. 11.20
Macroscopic photograph of a testicular benign mature teratoma

Fig. 11.21




Macroscopic photograph of a testicular benign mature teratoma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree