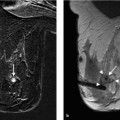
15 Guidelines for the Early Detection of Breast Cancer: Interventions and Excision Biopsy Condensed Version: Intervention Percutaneous core biopsy, vacuum-assisted biopsy (VAB), or open biopsy should be performed to obtain tissue samples for histologic assessment of ambiguous breast findings. Percutaneous interventions should be performed according to quality assurance recommendations. Fine needle aspiration biopsy (FNAB) cannot be recommended as a standard diagnostic method. Image-guided interventions are performed to obtain tissue samples for histologic verification and therapy planning from mammographic findings in the BI-RADS categories 4 and 5, and/or ultrasonographic findings in the BI-RADS categories 4 and 5, and/or magnetic resonance imaging (MRI) findings in the BI-RADS categories 4 and 5. Percutaneous large core needle biopsies (CNBs) should be ultrasound (US)-guided. At least four tissue samples using a ≤ 14-gauge needle should be harvested. When biopsying microcalcifications, stereotactic VAB technique should be employed. The VAB technique should also be employed for MRI-guided interventions. The stereotactic VAB procedure should be standardized. The needle access path and stroke margin must be documented. To document the correct needle position, the following are required: a scout image, the stereo pair images, and the prefire pair and postbiopsy pair images. At least 12 tissue samples should be harvested when using an 11-gauge needle. If using needles with a different caliber (8 to 11 gauge), the number of specimens harvested should yield an equivalent tissue volume. A specimen radiograph is required to verify correct tissue sampling and should be obtained using a magnification view. Successful sampling is confirmed when representative microcalcifications are detected in the tissue samples. Separating the samples with microcalcifications from those without often facilitates the detection of these for the pathologist. After VAB, a final mammographic documentation of the biopsied breast in two orthogonal planes should be performed. After each minimally invasive, image-guided intervention, correlation of imaging with histology should be performed to verify a representative biopsy. If the biopsy results are benign, a short-term follow-up in the respective imaging modality should be recommended in 6 months. Quality indicators with tentative reference values. Condensed Version: Excision Biopsy The preoperative localization of nonpalpable mammographic lesions should aim to place the localization wire so that it penetrates the target lesion and does not project more than 1 cm past the distal border. If it does not penetrate the lesion, it should be placed ≤ 1 cm from the target lesion. When localizing a nonmass lesion, however, two or more markers/wires can be placed at the surgically relevant borders without adhering to the 1-cm limit. The preoperative localization of nonpalpable breast lesions and the imaging confirmation of correct excision is indispensable. The surgical excision of lesions detected only by US should be verified by intraoperative specimen US. The excision specimen should be clearly marked by the surgeon to ensure proper orientation. It should be sent to the pathologist without further incisions after removal from the breast. A frozen section to attain an intraoperative statement about a lesion’s histology should only be performed in exceptional cases. Prerequisites for a frozen section on a breast specimen are: Quality indicators with tentative reference values. Needle biopsy techniques can be performed: The quality assurance of these procedures encompasses the following aspects: Guidance. Palpable findings can be percutaneously biopsied with or without sonographic guidance. For the biopsy of non-palpable imaging findings, the imaging method used for needle guidance should be chosen according to which method visualizes the lesion best and is the most reliable. This can be mammography (stereotactic), US, or MRI. Puncture method. The current standard biopsy methods are the large CNB and the VAB. FNAB is reserved for a very few specific indications. Which puncture method to choose is dependent upon the clinical constellation and the attainable accuracy of each method. In this regard, the puncture methods have significant differences: Quality assurance. The practice guidelines of the American College of Radiology (ACR) first included quality assurance measures for breast interventions in 1997. They currently apply to the equipment specifications and technical standards for diagnostic physics performance monitoring of imaging equipment, indications and contraindications for the performance of image-guided breast interventional procedures, and documentation (including image documentation) of the needle position, pathologic results, problems encountered, and follow-up recommendations. In addition, the ACR practice guidelines also include the minimum qualification requirements of the examiner, and the—albeit relatively small—number of interventions the examiner must perform per year to maintain his or her expertise. To promote the quality of core biopsy and VAB performance, studies pertaining to the optimal needle diameter and the number of specimens to be harvested have been performed and recommendations based on these studies exist in voluntary practice guidelines. In addition, a quality-assured procedural protocol has been developed specifically for the performance of MRI-guided interventions (VAB, preoperative localization; Breast Diagnostics Working Committee of the German Radiology Association). Independent of the biopsy method performed, it is always essential that histology be correlated with the diagnostic imaging (EU and ACR guidelines) to recognize biopsies that are not representative. Results that are discordant with the imaging assessment must be discussed in an interdisciplinary conference. A therapeutic recommendation can only be made after a representative biopsy has been ensured. If this is not the case, a repeat intervention or open biopsy must be performed. Thorough quality assurance on different levels is necessary for the performance of high-standard percutaneous interventions. High accuracy has been reported for CNB and VAB, but not, however, for FNAB. Quality standards for CNB and VAB have been developed. Guidelines Each ambiguous finding must be histologically verified using the most effective image-guidance method. Personnel requirements Physician: 1. Extensive knowledge and experience in breast diagnostic imaging, including the diagnostic image work-up of ambiguous findings, is a prerequisite for the reliable identification and localization of the significant lesion(s) on mammography in two orthogonal planes. 2. For the performance of stereotactic interventions: Performance of at least 30 stereotactic breast biopsy procedures under the supervision of a qualified physician, or performance of at least 10 stereotactic breast biopsy procedures under the supervision of a qualified company representative and at least 20 stereotactic breast biopsy procedures under the supervision of a qualified physician. Extensive knowledge of how to verify calibration (10 independently performed verification procedures) is also necessary. 3. For the performance of US-guided interventions: Extensive knowledge and experience in diagnostic breast US examination and performance of at least 30 US-guided breast biopsy procedures under the supervision of a qualified physician. 4. For the performance of MRI-guided interventions: Extensive knowledge and experience in diagnostic MR mammography and performance of at least 30 MRI-guided breast biopsy procedures under the supervision of a qualified physician. 5. For the maintenance of competence, the performance of at least 50 stereotactic-guided percutaneous biopsies per year, at least 50 US-guided percutaneous biopsies per year, and/or at least 50 MRI-guided VABs per year are recommended. Certified radiologist’s assistant: 1. Certification after having successfully completed an advanced academic program encompassing specialized training in breast diagnostics 2. For the performance of stereotactic-guided interventions: – extensive knowledge pertaining to the mammographic imaging technique, including image quality, breast positioning, radiation protection – vocational training in how to verify calibration (at least 10 verifications) and performance of at least 10 stereotactic breast biopsy procedures under the supervision of a an experienced radiologist’s assistant, a medical physicist/technologist, or engineer Technical quality control Certified radiologist’s assistant:
 The proportion of mammographic findings in the BI-RADS categories 4 and 5 with a correlating sonographic finding, which undergo a US-guided core biopsy that satisfies the quality standard specifications: ≥ 70%
The proportion of mammographic findings in the BI-RADS categories 4 and 5 with a correlating sonographic finding, which undergo a US-guided core biopsy that satisfies the quality standard specifications: ≥ 70%
 The proportion of mammographic findings that contain micro-calcifications, are in the BI-RADS categories 4 and 5, and are without a correlating sonographic finding, which undergo a stereotactic VAB that satisfies the quality standard specifications: ≥ 70%
The proportion of mammographic findings that contain micro-calcifications, are in the BI-RADS categories 4 and 5, and are without a correlating sonographic finding, which undergo a stereotactic VAB that satisfies the quality standard specifications: ≥ 70%
 The proportion of MR-mammographic findings in the BI-RADS categories 4 and 5 that are solely seen on MR mammography, which undergo an MRI-guided VAB that satisfies the quality standard specifications: ≥ 95%
The proportion of MR-mammographic findings in the BI-RADS categories 4 and 5 that are solely seen on MR mammography, which undergo an MRI-guided VAB that satisfies the quality standard specifications: ≥ 95%
 The proportion of mammographic findings in the BI-RADS categories 4 and 5, and/or sonographic findings in the BI-RADS categories 4 and 5, and/or MR-mammographic findings in the BI-RADS categories 4 and 5 that are histologically verified by an appropriate image-guided biopsy: ≥ 70%
The proportion of mammographic findings in the BI-RADS categories 4 and 5, and/or sonographic findings in the BI-RADS categories 4 and 5, and/or MR-mammographic findings in the BI-RADS categories 4 and 5 that are histologically verified by an appropriate image-guided biopsy: ≥ 70%
 The proportion of nonpalpable findings that are histologically verified preoperatively by mammographic-, sonographic-, or MRI-guided CNB or VAB: ≥ 70%
The proportion of nonpalpable findings that are histologically verified preoperatively by mammographic-, sonographic-, or MRI-guided CNB or VAB: ≥ 70%
 The proportion of palpable and nonpalpable findings that are histologically verified preoperatively by mammographic-, sonographic-, or MRI-guided CNB or VAB: ≥ 90%
The proportion of palpable and nonpalpable findings that are histologically verified preoperatively by mammographic-, sonographic-, or MRI-guided CNB or VAB: ≥ 90%
 The proportion of interventions for mammographic findings with microcalcifications in the BI-RADS categories 4 and 5 that have representative microcalcifications detected in the specimen radiograph: ≥ 95%
The proportion of interventions for mammographic findings with microcalcifications in the BI-RADS categories 4 and 5 that have representative microcalcifications detected in the specimen radiograph: ≥ 95%
 The proportion of cases in which surgical excision reveals a malignant finding after image-guided biopsy has yielded a benign finding (B classification 1 or 2) (false-negative): < 10%
The proportion of cases in which surgical excision reveals a malignant finding after image-guided biopsy has yielded a benign finding (B classification 1 or 2) (false-negative): < 10%
 The lesion is palpable intraoperatively and in the specimen.
The lesion is palpable intraoperatively and in the specimen.
 The lesion is large enough (generally > 10 mm).
The lesion is large enough (generally > 10 mm).
 The proportion of breast operations after preoperative wire localization in which the wire is placed ≤ 1 cm from the lesion border: ≥ 95%
The proportion of breast operations after preoperative wire localization in which the wire is placed ≤ 1 cm from the lesion border: ≥ 95%
 The proportion of breast operations after preoperative mammographic localization with intraoperative specimen radiography: ≥ 95%
The proportion of breast operations after preoperative mammographic localization with intraoperative specimen radiography: ≥ 95%
 The proportion of breast operations after preoperative US-guided localization with intraoperative specimen US: ≥ 95%
The proportion of breast operations after preoperative US-guided localization with intraoperative specimen US: ≥ 95%
 The proportion of excision specimens that are clearly topo-graphically marked by the surgeon: ≥ 95%
The proportion of excision specimens that are clearly topo-graphically marked by the surgeon: ≥ 95%
Evidence-Based Medicine: Interventional Techniques
Basic Principles
 to verify the presence of a suspected malignancy (before neoadjuvant chemotherapy and for surgical planning)
to verify the presence of a suspected malignancy (before neoadjuvant chemotherapy and for surgical planning)
 for minimally invasive histologic work-up of ambiguous and nonpalpable breast findings
for minimally invasive histologic work-up of ambiguous and nonpalpable breast findings
 the technique used (targeting the lesion, harvesting specimens)
the technique used (targeting the lesion, harvesting specimens)
 the processing and assessment of the pathologic/cytologic specimen
the processing and assessment of the pathologic/cytologic specimen
 the appraisal of pathologic results and the resultant therapy planning
the appraisal of pathologic results and the resultant therapy planning
Choice of Method
 The FNAB cannot be recommended as a standard biopsy method (level of evidence [LOE] 3b–3a). Its application should be limited to special cases (complicated cysts, lymph nodes) where specific cytology results can be expected.
The FNAB cannot be recommended as a standard biopsy method (level of evidence [LOE] 3b–3a). Its application should be limited to special cases (complicated cysts, lymph nodes) where specific cytology results can be expected.
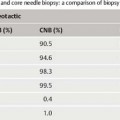
 Using a standardized technique (see below), the large CNB reaches a sensitivity of 85 to 98% (LOE 3b–3a).
Using a standardized technique (see below), the large CNB reaches a sensitivity of 85 to 98% (LOE 3b–3a).
 VAB reaches a sensitivity of over 98% using a standardized technique (LOE 3b–3a).
VAB reaches a sensitivity of over 98% using a standardized technique (LOE 3b–3a).
Summary
Structural Quality: Interventional Techniques
Percutaneous Biopsy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree