5
Gynecologic Brachytherapy
Larissa J. Lee, Antonio L. Damato, and Akila N. Viswanathan
Brachytherapy is an integral component in the treatment of gynecologic cancer, and one that requires a thorough understanding of radiobiology, physics, imaging, and surgical techniques. By taking advantage of the inverse square law, brachytherapy has the unique ability to selectively deliver a curative dose to tumors of the cervix, endometrium, and vagina, while minimizing the delivered dose to adjacent critical organs, such as the rectum, sigmoid, bladder, and small bowel. The gynecologic organs, specifically the uterus, uterine cervix, and vagina, are hollow viscera. The uterus and cervix have a relatively high tolerance for radiation, which allows for placement of the brachytherapy sources in direct contact with the tumor. Although the uterus, cervix, and vagina are highly mobile, gynecologic brachytherapy applicators remain relatively fixed within the target despite patient or organ motion, allowing for dose escalation with rapid dose falloff and lower integral radiation doses when compared to highly conformal external beam techniques. Furthermore, patients who receive higher doses of external beam radiotherapy (EBRT) instead of intracavitary brachytherapy have higher rates of local failure, shorter survival times, and increased complications (1−7).
EVALUATION OF GYNECOLOGIC CANCERS
Staging for cervix cancer relies on clinical evaluation, according to the International Federation of Gynecology and Obstetrics (FIGO) system, which allows for chest radiography, bimanual and rectovaginal examination under anesthesia, cystoscopy and/or proctoscopy (in patients with urinary or rectal symptoms), and IV pyelography to evaluate for hydronephrosis (8,9). When available, FIGO encourages the use of advanced imaging modalities, such as CT, MRI, and PET, to guide decisions regarding therapy. However, the imaging findings may not be used to determine disease stage, in order to preserve the FIGO system. For patients who are candidates for brachytherapy, in addition to a thorough gynecologic examination, cross-sectional imaging is recommended at the time of diagnosis, immediately before brachytherapy, and ideally at the time of each insertion, in order to assist in brachytherapy planning and delivery.
Given its superior soft-tissue resolution, MRI is particularly useful in delineating normal female pelvic anatomy, as well as the size and extent of uterine, cervical, and vaginal tumors (Figure 5.1). MRI is more sensitive than physical examination in estimating tumor size and assessing paravaginal or parametrial involvement in patients with cervical or vaginal cancer (10−13). T2-weighted images are particularly helpful in identifying parametrial extension with disruption of the normally dark cervical stromal ring, as the gross tumor volume generally appears bright or isointense (low T1, high T2 signal) compared to the normal cervix (low T1, low T2). Direct extension of tumor to the pelvic sidewall, bladder, and/or rectum may also be reliably determined by MRI and obviate the need for cystoscopy and proctoscopy (14,15). The sensitivity of MRI for detecting deep myometrial invasion (greater than 50%) in patients with uterine cancer is approximately 90% (16,17). Detailed views of uterine and cervical anatomy may also guide subsequent instrumentation of the uterine canal for dilatation and tandem placement. The acquisition of serial MRIs with quantification of tumor regression rates has been shown to be prognostic during definitive radiotherapy for cervical cancer (18−20). Furthermore, the use of three-dimensional (3D) imaging results in more accurate delineation of the tumor, cervix, uterus, and organs at risk at the time of brachytherapy (21). CT imaging, which is a less sensitive but more widely available tool, can estimate cervical diameter, confirm applicator positioning, and identify organs at risk. CT may also be used for image-guided intracavitary or interstitial application, with an MRI performed before brachytherapy or at the time of the first application to assess tumor regression for brachytherapy planning purposes (22,23).

Figure 5.1 (A) Axial MR image of gross tumor within the cervix (high T2 signal) with disruption of the normal dark cervical stroma (low T2). (B) Sagittal image of a large primary cervical cancer with complete replacement of cervix, uterine extension, and retroverted position. (C) Sagittal image of a primary uterine carcinoma (high T2 signal) with loss of endometrial stripe (low T2) and full thickness myometrial invasion.
PET using 2-[fluorine 18]-fluoro-2-deoxy-d-glucose (FDG) has become the standard imaging tool when available for the detection of nodal or distant metastases for cervical and vaginal cancer, and may have clinical utility for high-grade uterine cancer (24−27). Pelvic and/or paraaortic lymph node involvement as visualized by PET may be used to tailor the extent of the radiation treatment field as well as to determine the boost dose to pathologically enlarged or FDG-avid nodes. Furthermore, treatment response at the time of a 3 month post-therapy PET scan has been shown to be prognostic for recurrence and survival (28).
BRACHYTHERAPY MODALITIES
Low dose rate (LDR) brachytherapy has been a mainstay in the definitive treatment of cervical cancer for over a century, and the specifics of LDR application, including source loading patterns, treatment time, and total delivered dose, have been validated by long-standing clinical experience. With the introduction of remote afterloaders and new radionuclides, high dose rate (HDR) and pulsed dose rate (PDR) brachytherapy allow for dose optimization with a stepping source and variable dwell times. The average dose rate for PDR is 0.4 to 0.6 Gy per hour, which approximates the average dose rate of LDR, while using an iridium-192 (192I) source with a strength of less than 1 Curie. The dose rate for HDR typically exceeds 12 Gy per hour (29). To allow for normal tissue repair, a minimum of 6 hours is recommended between HDR fractions (30−34). A meta-analysis of four randomized trials of LDR versus HDR brachytherapy has demonstrated the equivalence of these modalities for cervical cancer (35).
Table 5.1 Comparison of HDR, LDR, and PDR brachytherapy for cervical cancer
| Advantages | Disadvantages |
HDR | • Outpatient treatment • No radiation exposure to staff • Short administration time • Optimization with stepping source and variable dwell times | • Multiple brachytherapy fractions • Theoretic radiobiologic disadvantage with large dose per fraction |
LDR | • Maximum two insertions • Standardized delivered dose and treatment plan • Radiobiologic advantage of sublethal damage repair | • Inpatient treatment • Prolonged bed rest • Need for inpatient pain control • Radiation exposure to staff • Limited by available sources |
PDR | • Maximum two insertions • No radiation exposure to staff • Optimization with stepping source and variable dwell times • Radiobiologic advantage of sublethal damage repair | • Inpatient treatment • Prolonged bed rest • Need for inpatient pain control |
HDR, high dose rate; LDR, low dose rate; PDR, pulsed dose rate.
The use of HDR brachytherapy for cervical cancer has increased substantially in the United States and internationally over the last decade. In a Quality Research in Radiation Oncology (QRRO) study, the proportion of surveyed facilities that used HDR increased from 13% in 1996–1999 to 62% in 2007–2009 (36). Surveys from the American Brachytherapy Society (ABS [2007]) and the Gynecologic Cancer Intergroup (2009) reported that 85% of brachytherapy practitioners use HDR (37,38). Other advantages for HDR and PDR brachytherapy are further summarized in Table 5.1. Dose equivalence between HDR and LDR may be estimated using the biological effective dose (BED) formula and the equivalent dose in 2 Gy per fraction (EQD2) formula; calculation sheets are available at http://www.americanbrachytherapy.org/guidelines/index.cfm.
In select centers, HDR, PDR, and/or LDR brachytherapy are available, which allows for greater flexibility in the selection of the brachytherapy modality. For cervical cancer, HDR may be ideal for patients with a small volume of residual disease at the time of brachytherapy, in whom sufficient packing and good implant geometry displace the bladder and rectum from the high-dose region. Certain rare histologies, such as melanoma and sarcoma, may benefit from a large fraction size given their relative radioresistance. Patients at an increased risk of a thrombotic event during an inpatient admission may be more appropriate candidates for outpatient HDR treatment. LDR or PDR may be preferable in patients with a history of prior radiation, as reirradiation increases the risk of complication, such that the radiobiologic advantage of LDR or PDR outweighs the convenience of HDR (39). In summary, HDR, PDR, and LDR brachytherapy are considered equivalent in terms of outcome and complication risk when carefully applied and individualized based on the needs of the patient and pertinent clinical history.
LOCALLY ADVANCED CERVICAL CANCER
Case 5.1 FIGO Stage IIA1 Cervical Cancer
A 42-year-old woman with intermenstrual bleeding presented for gynecologic evaluation, and clinical examination revealed an abnormal-appearing cervix measuring 3 cm with an erythematous lesion extending to the anterior and posterior vaginal walls (Figure 5.2). There was no parametrial involvement on rectovaginal examination. Cervical biopsy showed endocervical adenocarcinoma and her disease was staged as FIGO IIA1. PET/CT at diagnosis showed intense FDG uptake in the cervix and no evidence of nodal or distant metastases. Pelvic MRI showed a 4 cm cervical mass with vaginal wall thickening (Figure 5.3). She received EBRT to the pelvis with a dose of 45 Gy in 25 fractions delivered with concurrent weekly cisplatin (40 mg/m2). Over the course of treatment, she developed diarrhea, which was managed with adherence to a low-fiber diet, loperamide and diphenoxylate/atropine as needed, and Metamucil.
Prior to brachytherapy, examination revealed robust interval response in the size of the cervical mass, with no appreciable vaginal involvement and no active bleeding (Figure 5.2). Preoperative evaluation included an anesthesia assessment; laboratory studies, including complete blood count and comprehensive metabolic panel; and obtaining consent for the procedure and radiation. The implant was performed under general anesthesia in a brachytherapy suite. Prophylaxis for deep vein thrombosis included placement of pneumatic boots and compression stockings for the duration of the procedure. Examination under anesthesia confirmed the aforementioned findings. Rectal contrast was instilled using a flexible rubber tube. Following sterile preparation and draping, a Foley catheter was placed and 120 mL of normal saline was instilled into the bladder for improved ultrasound visualization. A uterine sound was used to measure the uterine length under ultrasound guidance. A 6 cm tandem and 2.6 cm ring applicator were selected based on the uterine length, small tumor size, and relative effacement of the cervix. Following serial dilation, the tandem was placed and its position within the uterus was confirmed by ultrasound (Figure 5.4). The ring was secured to the tandem with its position flush against the cervix. Balloon-based packing was used to displace the rectum posteriorly and the bladder anteriorly from the applicator. The bladder was emptied and filled with 30 mL of diluted contrast.

Figure 5.2 Clinical photographs of FIGO Stage IIA1 cervical cancer (A) at diagnosis and (B) prior to the first brachytherapy implant. FIGO, International Federation of Gynecology and Obstetrics.

Figure 5.3 T2-weighted images in (A) axial and (B) sagittal planes of pelvic MRI from diagnosis for FIGO Stage IIA1 cervical cancer. FIGO, International Federation of Gynecology and Obstetrics.
The applicator was immobilized with a brachytherapy board and CT images were acquired for treatment planning. The radiation oncologist contoured the bladder, rectum, sigmoid, and small bowel adjacent to the applicator. The high-risk clinical target volume (HR-CTV) encompassed the entire cervix based on examination findings at the time of brachytherapy (Figure 5.5). With a standard load and planned prescription dose of 5.5 Gy for five fractions (total 27.5 Gy), manual optimization was performed to limit dose to the adjacent normal tissue structures while maintaining coverage of the HR-CTV. Dose–volume histogram (DVH) metrics for fraction 1 were D2cc (D2cc is generally being replaced by D2cm3 internationally) bladder: 2.9 Gy; D2cc rectum: 2.2 Gy; D2cc sigmoid: 1.0 Gy; D2cc small bowel: 4.2 Gy; and D90 for HR-CTV: 5.7 Gy. For the subsequent fractions, the small bowel was noted to be highly mobile and dose was further reduced by fixed bladder filling prior to simulation and treatment (range: 1.7–3.5 Gy per fraction). On evaluation of the cumulative DVH metrics using EQD2, D90 for HR-CTV was 81.6 Gy (102%); D2cc rectum 57.5 Gy (planning aim < 75 Gy); D2cc bladder 72 Gy (planning aim < 90 Gy); D2cc sigmoid 55 Gy (planning aim < 75 Gy); and D2cc small bowel 65 Gy (planning aim < 65 Gy). The patient tolerated brachytherapy well without complication. Posttreatment surveillance PET-CT at 3 months showed no evidence of persistent or metastatic disease. Her clinical examination and Pap smear were within normal limits at most recent follow-up, 18 months after completion of treatment.

Figure 5.4 Ultrasound-guided tandem placement for FIGO Stage IIA1 cervical cancer. FIGO, International Federation of Gynecology and Obstetrics.

Figure 5.5 Coronal (A) and sagittal (B) views of a tandem and ring treatment plan. Organs at risk are contoured as follows: rectum in brown, sigmoid in blue, bowel in green, and bladder in yellow. The HR-CTV is shown in red. The bowel in green was displaced at subsequent fractions by bladder filling. The 100% isodose line is shown in yellow. HR-CTV, high-risk clinical target volume.
A 60-year-old woman presented with pelvic and right flank pain, and evaluation by her primary care physician was concerning for a pelvic mass. Examination under anesthesia revealed a large barrel-shaped cervix extending to the right pelvic sidewall and involving the rectovaginal septum. On cystoscopy, the right ureter was obstructed without frank bladder involvement and percutaneous nephrostomy was required. Biopsy of the cervical mass confirmed squamous cell carcinoma. CT imaging revealed a 6 × 5.5 cm mass arising from the cervix and involving the bladder base with right-sided ureteral obstruction and dilatation leading to hydronephrosis (Figure 5.6). PET-CT showed a highly FDG-avid pelvic mass without nodal or distant metastatic spread. The patient received concurrent chemoradiotherapy to 45 Gy with weekly cisplatin as previously described, complicated by fatigue, nausea, and diarrhea requiring IV hydration and electrolyte repletion. On prebrachytherapy examination, the barrel cervix measured 4 cm with palpable firmness involving the anterior vaginal wall and rectovaginal septum and extending to the right pelvic sidewall. MRI was obtained for brachytherapy planning purposes, which revealed a 3.6 cm partially exophytic right cervical mass with right pelvic sidewall extension, and invasion of the bladder wall, upper vagina, and anteriolateral rectal wall (Figure 5.7). There was no hydronephrosis or enlarged lymph nodes.
Due to the extent of disease at presentation and prior to brachytherapy, interstitial implantation was planned under MR guidance. The patient had significant improvement in the acute treatment-related side effects 1 week after the completion of EBRT. She was provided instructions for a bowel preparation with stool softeners for 2 days prior to the implant and a clear liquid diet and enema 1 day prior to the planned procedure. Following anesthesia clearance, the procedure was performed under general anesthesia with epidural placement for the duration of the hospitalization. Deep vein thrombosis prophylaxis included administration of subcutaneous low-molecular-weight heparin. Antibiotics and antidiarrheal medication were also given while the patient was on bed rest.
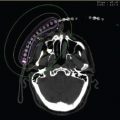
Following standard procedural preparation, a tandem was placed in the uterus under ultrasound guidance. A split vaginal obturator was advanced over the tandem to the level of the cervix and stabilized with placement of a perineal interstitial template stitched at four corners to the perineal skin. The patient was subsequently transferred to an MR operating suite where catheters were serially placed through the template. The catheter position was evaluated by serial MRI acquisition and adjusted as needed to optimize tumor coverage (Figure 5.8). The posterior bladder and anterior rectal walls were implanted at sites of radiographic involvement.

Figure 5.6 Baseline CT imaging for FIGO Stage IIIB cervical cancer with right pelvic sidewall extension and hydronephrosis (A: sagittal, B: axial). FIGO, International Federation of Gynecology and Obstetrics.

Figure 5.7 Prebrachytherapy MRI with residual gross disease involving right-sided parametrial and uterosacral ligaments. The endocervix is visible centrally as a T2-dark ring.
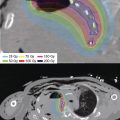
For brachytherapy planning, CT imaging was obtained with dummy strand placement to confirm catheter position for applicator reconstruction. The HR-CTV was contoured to encompass the residual disease at the time of brachytherapy based on MR and examination findings. HDR brachytherapy was delivered with a dose of 3.75 Gy for nine fractions for a cumulative tumor dose in EQD2 of 83 Gy (Figure 5.9). Treatment was delivered twice daily with an interfraction interval of at least 6 hours. The cumulative EQD2 doses for D2cc bladder and rectum were 78.3 Gy and 81 Gy, respectively. Surveillance MR and PET-CT imaging were equivocal, and subsequent biopsies performed under anesthesia were negative for tumor. She has required permanent placement of a right ureteral stent for persistent stricture.
Figure 5.8 Intraoperative axial MR image with placement of an intrauterine tandem and interstitial catheters in the right-sided pelvic sidewall disease.

Figure 5.9 Dose distribution of the MR-guided interstitial treatment plan in coronal, sagittal, and axial planes. The HR-CTV is contoured in red, the bladder in yellow, the rectum in brown, and the sigmoid in blue. The 100% isodose line is shown in yellow. HR-CTV, high-risk clinical target volume.
Imaging
Movement of the patient should be minimized such that the simulation conditions are reproduced at the time of brachytherapy. For HDR applications, the tandem is rigidly immobilized in a midline position to a brachytherapy board at simulation and treatment. Approximately 30–60 mL of diluted (1:10) hypaque contrast should be instilled into an emptied bladder with a clamped Foley catheter, which is subsequently unclamped after imaging. When available, IV contrast may help visualize the uterine vessels and determine the superior border of the cervix. CT- or MR-compatible dummy strands should be placed into the applicator and/or catheters for visualization and applicator reconstruction. During CT simulation, transverse images of the pelvis are acquired in 1 to 5 mm increments and digitally reconstructed radiographs (DRRs) are generated. Implant geometry and uterine perforation may be assessed on axial as well as reconstructed sagittal and coronal reconstructed images. If inadequate, the implant should be repositioned and repacked before the final series of images is obtained for treatment planning. Fusion software should be available if multiple imaging modalities are used for contouring and planning.
ENDOMETRIAL CANCER
Adjuvant Vaginal Brachytherapy
Adjuvant vaginal vault brachytherapy has become a mainstay in the postoperative treatment of early-stage endometrial cancer to prevent local recurrence (14). In the randomized Post Operative Radiation Therapy in Endometrial Carcinoma (PORTEC)-2 study of early-stage, high-intermediate-risk endometrial cancer, vaginal brachytherapy was associated with equivalent local control and survival rates, and less associated gastrointestinal toxicity, when compared to pelvic EBRT (40). Patient selection for adjuvant vaginal brachytherapy is based on patient and intrauterine risk factors following hysterectomy, which include patient age, tumor grade, lymphovascular invasion, tumor size, lower uterine segment or cervical involvement, and the extent of surgical staging (41,42). As the rate of local-regional recurrence is relatively low (10%–15%) for intermediate-risk disease without an associated survival benefit (Gynecologic Oncology Group [GOG]-99, PORTEC), the recommendation for adjuvant treatment should be discussed with the patient in the context of the associated risks and benefits. Following adjuvant vaginal brachytherapy, the rate of vaginal recurrence is extremely low, ranging from 0% to 3% in retrospective and prospective studies, and reported grade 3 to 4 toxicities are as low as 0% to 2% and are dependent on dose-fractionation schedules (43).
Prior to brachytherapy and at least 4 weeks after hysterectomy, the physician should perform a thorough examination of the vagina with careful attention to the colpotomy site to assess healing. If recurrence is suspected, biopsy should be performed. The most commonly used applicator is the vaginal cylinder, which is available in various lengths and diameters that range from 2 to 4 cm. At the time of applicator fitting, the cylinder with the largest diameter that can be inserted without significant patient discomfort should be used to improve cylinder annealing and to provide the physical advantage of a more gentle dose gradient. The cylinder should appose the vaginal apex, fornices, and full vaginal length to deliver adequate dose to the mucosa. In contrast to the commonly used single-channel cylinder, multichannel cylinders are available with a central channel and peripheral catheter positions that allow for differential loading and shaped isodose distributions that may reduce bladder and rectal doses without compromising target coverage (41). Other practitioners prefer the use of ovoids, although only the upper vagina can be treated and greater separation of the ovoids may lead to central under-dosing. Applicator positioning should be confirmed before treatment with fiducial placement on radiograph, CT, or MRI if available. CT simulation also allows for assessment of the vaginal length determined clinically, cylinder positioning, and evaluation of air gaps (Figure 5.10). For HDR brachytherapy, the applicator should be immobilized with a stabilization board on the treatment table. LDR cylinder brachytherapy requires inpatient hospitalization and is rarely used. Due to source anisotropy, LDR cylinders do not provide a uniform dose rate to the vaginal surface, which may be particularly pronounced over the cylinder dome surface.
The dose fractionation regimens for HDR vaginal cylinder brachytherapy deliver approximately 60 Gy in EQD2 to the vaginal surface, although novel low-dose fractionation schemes have been reported with good results (43,44). Common regimens include 7 Gy × 3 fractions prescribed to 0.5 mm depth (PORTEC-2 study) and 6 Gy × 5 fractions (MD Anderson Cancer Center) or 4 Gy × 6 fractions (Dana-Farber/Brigham and Women’s Hospital) prescribed to the vaginal or cylinder surface. The vaginal treatment length is controversial, although in most cases dose is prescribed to the proximal 3 to 5 cm of the vagina, as most failures occur at the vaginal apex (45). Treatment of the full vaginal length may be considered in the presence of risk factors such as lymphovascular invasion or high-risk histology (uterine serous or clear cell carcinoma). HDR fractions are delivered in multiple (two to four) fractions per week. Details of dose prescription and cylinder optimization, including optimization points and method, the number of dwell positions, relative dwell weights, and isodose distribution, should be clearly documented. When available, CT simulation allows for the identification of organs at risk, although contouring the rectum and bladder is not routinely performed given the relatively low delivered doses and low complication rates (46). Similarly, an individualized treatment plan does not need to be recalculated for each fraction with the use of fixed geometry applicators. Following cylinder treatment, the patient should be counseled on the importance of routine vaginal dilation to prevent stenosis, although the risks of vaginal shortening, mucosal atrophy, and bleeding may be reduced with a low-dose fractionation scheme (43).

Figure 5.10 CT simulation to assess vaginal length and cylinder positioning and diameter prior to treatment.
Medically Inoperable Endometrial Cancer
Case 5.3 Clinical FIGO Stage 1A Endometrial Cancer
A 54-year-old woman with morbid obesity (BMI of 60) was evaluated for postmenopausal vaginal bleeding. Pelvic examination was limited due to body habitus, although it showed no evidence of cervical or vaginal disease. Endometrial biopsy was performed and pathology showed grade 1 endometrioid adenocarcinoma. In the setting of heavy bleeding, she was started on a progestin and consented for robotic hysterectomy and salpingo-oophorectomy. She developed acute onset of shortness of breath, and CT imaging revealed bilateral pulmonary emboli with right heart strain on echocardiogram. She was treated with catheter-based lytic therapy and transitioned to low-molecular-weight heparin. She was no longer considered an appropriate surgical candidate due to therapeutic anticoagulation.
Staging abdominal and pelvic CT with IV/PO contrast showed a heterogeneous endometrial stripe and fatty liver change, but no nodal or distant metastatic disease. Pelvic MRI showed an abnormal signal within the endometrial cavity. The depth of myometrial invasion was difficult to assess, but was not greater than 50% (Figure 5.11). The estimated risk of nodal involvement with grade 1 disease and less than 50% myometrial invasion was considered low. Therefore, she received brachytherapy alone as definitive treatment. Anticoagulation management was reviewed with her cardiologist with the plan to hold the heparin dose the morning of the procedure and resume it during hospitalization. Following preprocedural and anesthesia clearance, she underwent placement of a Martinez double tandem under general anesthesia. As she was not an epidural candidate given the need for therapeutic anticoagulation, patient-controlled analgesia (PCA) with IV hydromorphone was used for pain control. The brachytherapy prescription was a nominal HDR dose of 44 Gy in eight fractions (5.5 Gy per fraction) delivered twice daily over 5 days. Dose was prescribed to the clinical target volume (CTV) that encompassed the entire uterus, cervix, and upper vagina (Figure 5.12). The uninvolved vagina received 32 Gy in eight fractions. Evaluation of the dose distribution showed that the surface of the endometrium received at least 200% of the prescribed dose. The cumulative dose metrics in EQD2 were D90 57.7 Gy (102%, prescription 56.8 Gy), D2cc rectum 11.0 Gy, D2cc bladder 58.3 Gy, and D2cc sigmoid 36.9 Gy. She tolerated treatment well with no evidence of recurrence by clinical examination or Pap smear and no late complications of treatment.

Figure 5.11 T2-weighted pelvic MR images in (A) axial and (B) sagittal planes for medically inoperable patient with grade 1 endometrioid adenocarcinoma of the uterus.

Figure 5.12 Isodose distribution for Martinez double tandem and cylinder treatment in (A) sagittal and (B) coronal planes. The 100% isodose line is shown in yellow and 200% in red.
Medically inoperable endometrial cancer is a rare scenario, as less than 5% of women present with significant medical comorbidity that precludes hysterectomy as primary treatment. Patients who are unable to undergo surgery are staged by the 1971 FIGO staging system based on the length of the uterine cavity, the presence of cervical involvement and/or extrauterine spread (47). When feasible, staging CT is performed to assess for nodal and distant metastasis; pelvic MRI is a sensitive tool for detecting deep myometrial invasion (48). In select patients with early-stage, low-grade disease (FIGO 1A, grade 1–2), brachytherapy alone may be considered, as the risk of pelvic lymph node involvement is low. A tandem-based applicator system (tandem and ovoids, tandem and ring, or tandem and cylinder) is acceptable for intrauterine application, although double and triple tandem applicators, which are available with or without a cylinder component, may provide superior uterine coverage while meeting bowel and bladder constraints (49). Simon-Heyman capsules are available in both HDR and LDR versions, although dosimetry may not be superior to that of a double or triple tandem applicator. The CTV should encompass the entire uterus, cervix, and upper 3 to 5 cm of the vagina. For brachytherapy alone, various dose fractionation schemes have been published (such as 7.5 Gy × 5 fractions or 8.5 Gy × 4 fractions) to deliver an equivalent dose of 54 Gy at 2 cm depth from a single tandem (50). These series show excellent local control and low toxicity rates (51).
For patients with deep myometrial invasion, high tumor grade or cervical/extrauterine spread, an external beam dose of 45 Gy in 25 fractions may be followed by five intracavitary brachytherapy applications of 5 to 5.5 Gy for a cumulative dose of 75.5 to 79.8 Gy in EQD2. The treatment isodose distribution should cover the outer surface of the uterus while maintaining standard dose constraints for the organs at risk (D2cc for bladder, rectum, and sigmoid).