271CHAPTER 17
Gynecologic SBRT
Stereotactic body radiation therapy (SBRT) clinical trials for gynecologic indications are few in number (1, 2). This is in spite of the observation that the persistence or recurrence of gynecologic malignancies (i.e., ovary, uterus, cervix, and vulva) following first-line treatments is a leading cause of cancer-related death among women worldwide (3). In retrospective review, persistent or recurrent gynecologic tumors abut formerly irradiated tissues or chemotherapy-taxed hematopoietic marrow in as many as 40% of patients (4, 5). When surgery, chemotherapy, or radiation therapy have already been attempted, selection of appropriate second- or third-line intervention becomes vital, as additional treatment may also bring about harm to the patient. As such, women with persistent or recurrent metastatic gynecologic malignancies have unmet therapeutic needs, especially when these malignancies are chemorefractory or have been irradiated earlier. Gynecologic SBRT meets at least one of the unmet needs by offering a noninvasive way of controlling disease at sites in which conventional surgery, chemotherapy, or radiation therapy are likely incapable of doing so.
Gynecologic SBRT demands sophisticated planning features critical to maximizing radiation dose delivery performance. Cancer care delivery research and clinical trials have studied (a) motion management of the patient on the tabletop, (b) motion management of the internal SBRT target because of physiologic motion, (c) high-level SBRT treatment planning and software performance, and (d) challenges of “real-time” beam-on on-board image capture for quality radiation dose delivery. Retrospective research and clinical trial efforts have been spent to best understand merits and demerits of hypofractionated, high-dose (≥8 Gy) radiation treatments that are intended to safely shorten weeks of conventional radiation therapy to as few as 3 days. Currently, gynecologic SBRT involves clinical radiation accelerators fitted to industrial robots (CyberKnife® [1, 2, 6–9]), to helical mounts (TomoTherapyTM or Vero [10–14]), or to conventional image-guided teletherapy machines (linear accelerator [linac]-based radiation therapy [15–18]).
This chapter offers a management synopsis of SBRT for persistent or recurrent metastatic gynecologic malignancies, and an up-to-date review of SBRT’s activity relative to disease control.
TREATMENT INDICATIONS
Treatment indications for gynecologic SBRT continue to evolve with clinical experience. So far, phase II clinical trial evidence offers support for the treatment of women whose persistent or recurrent metastatic ovarian, uterine, cervical, or vulvar disease (a) is chemorefractory or (b) has been irradiated earlier (1). Phase I clinical trial data support gynecologic SBRT–chemotherapy in combination (2). Anecdotal gynecologic SBRT indications include medically inoperable disease (15, 16) or as a dose boost when conventional radiation therapy and brachytherapy are conceded (10, 11, 17, 18). Each needs further investigation before being considered mainstream.
272PATIENT SELECTION
Patient candidate selection for gynecologic SBRT remains arbitrary. This is due in part to the relative dearth of level I evidence in this area. Consequently, investigators are still exploring the exact indications, prescriptions, and timetables for optimal gynecologic SBRT treatment. For now, gynecologic SBRT clinical trials usually recruit patient candidates who are older than 18 years, have an Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1, or 2 (i.e., relatively fit, attend their own activities of daily living), and have four or less (≤4) intended sites of SBRT treatment. There are no restrictions for gynecologic SBRT target volume when used as monotherapy (1); in gynecologic SBRT–chemotherapy combinations (2), a conservative 160 cm3 or less volume for each individual SBRT target is recommended (i.e., up to four 160 cm3 or less targets could be treated in one gynecologic SBRT session overlapping a chemotherapy cycle). Gynecologic SBRT has not yet been limited by prior chemotherapy exposure or by prior radiation therapy dose (1, 2).
WHY SBRT?
SBRT permits the use of a noninvasive radiation therapy treatment that increases the likelihood of local disease control for persistent or recurrent metastatic tumors. It has demonstrated durable clinical benefit in women with persistent or recurrent metastatic gynecologic malignancies (1). A rationale for gynecologic SBRT arises out of the notion that gynecologic tumors are particularly sensitive to radiation therapy–related nuclear and mitochondrial DNA damage, demonstrating steep drop-off of the radiation dose–cell survival curve at an ablative dose of 8 Gy (19). Indeed, ablative 8 Gy or higher radiation therapy doses lead to nuclear and mitochondrial DNA damage in targeted cells, increasing the difficulty of cell repair and possibly proving lethal to targeted cells (20). If gynecologic SBRT can deliver spot-on accurate and precise radiation therapy dose without unwarranted normal tissue injury, control of gynecologic SBRT-targeted disease is plausible.
SIMULATION
Video-supplemented guides are accessible for gynecologic SBRT simulation logistics for targets in the abdomen (21) and in the lung (22). Currently, before gynecologic SBRT simulation, it is recommended that at least one long (0.75 mm × 20 mm) gold-coated fiducial be placed through the SBRT target center of mass of each tumor intended for SBRT treatment.
Although there are likely to be numerous simulation setup positions, clinical trial standard operating procedures align patients in a head-first supine position for a contrast CT scan that captures anatomy from the orbitomeatal line through the upper thighs during quiet free breathing. The arms are supported over the head. For deep pelvic gynecologic SBRT, a comfortable full-bladder technique is recommended to push small bowel away from disease targets. Otherwise, an empty bladder technique is used. Two-pin localized ankle or knee sponges and head supports are used for patient comfort and consistency of setup over the 3 consecutive days of SBRT treatment. For breathing motion management, machine platforms may use skin-surface infrared markers or vests with integrated light-emitting diodes and, thus, should be included at simulation. Other in-room devices, such as video, infrared, or beam’s-eye-view cameras, and their relative alignment to the patient, should be well thought out prior to simulation, as these aid motion management. Because of video monitoring and engineering features such as motion synchrony by robotics or linac pan-and-tilt, rigid immobilization of the patient on the tabletop occurs infrequently for gynecologic SBRT.
Advanced radiology studies frequently enhance accuracy of gynecologic SBRT delivery for gynecologic recurrences (or applications). A 2-[18F] fluoro-2-deoxy-D-glucose PET scan captured in the same SBRT simulation position affords metabolic and anatomic overlay. An alternative or added imaging study such as MRI scan could be obtained for further anatomic detail. The gynecologic SBRT clinical trials recommended that both treating radiation oncologists and gynecologic oncologists contour on CT images, adding metabolic (i.e., PET) or anatomic (i.e., MRI) detail when drawing the SBRT primary gross target volumes (GTVp). In prior work, it was decided that a 40% thresholded tumor PET maximum standard uptake value enclosed clinically suspicious target margins and was labeled as another intended SBRT target volume (named clinical target volume on PET [CTVpet]) (23). For gynecologic SBRT planning, an internal target volume (ITV) represented the sum of the CT GTVp plus CTVpet contours.
273TARGET DELINEATION
Simulation CT images, in-room laser-aligned tattoo and breathing motion sensor data (if acquired), and SBRT platform parameters are all imported into the SBRT treatment planning software prior to target and normal anatomy delineation. Contours for normal anatomy are determined. GTVp targets (i.e., up to four targets for one SBRT treatment session) and corresponding MRI GTVp or CTVpet contours (as described earlier) are compiled into ITVs. To reduce a chance for geographic miss, gynecologic SBRT clinical trials enlarged ITVs in all dimensions by 5 mm for a SBRT planning target volume (PTV). SBRT dose planning occurred on the referent free-breathing phase CT scan.
An alternative approach to ITV contouring involves segmenting tumor and normal anatomy position among three types of CT scans: (a) free breathing; (b) comfortable maximum inspiration; and (c) comfortable maximum expiration phase. Here, the obvious corresponding GTVp volumes are labeled free-breathing (GTVfb), inspiration (GTVi), and expiration (GTVe). The ITV, therefore, is computed as the sum of the GTVfb, GTVi, and GTVe contours. For many cases, a CTVpet adds into the ITV. Again, a composite ITV is enlarged by 5 mm in every direction for a final PTV. For this alternative approach, radiation planning occurs on the free-breathing phase CT scan.
In a clinical scenario of when a thoracic target is considered for gynecologic SBRT, it has been recommended that normal anatomy contours of the trachea and right and left mainstem bronchi be enlarged by an additional 3 mm in all directions. These expanded trachea and bronchial contours are assigned a high-priority planning constraint to avoid late toxicity in airway fibrosis.
TREATMENT PLAN EVALUATION
The treating radiation oncologist must assess gynecologic SBRT treatment plans slice by slice. Each SBRT platform’s high-technology planning software permits three- or four-dimensional treatment plan rendering, making review of radiation beam orientation relative to radiation dose–volume histograms practical. Clinical trials have provided guidance for SBRT plan evaluation and acceptable plan deviations as listed in Table 17.1.

274DOSE CONSTRAINTS TO CRITICAL STRUCTURES
As the majority of gynecologic SBRT cases exist in the setting of reirradiation, abiding by cumulative tissue tolerance dose constraints is paramount. The goal is to deliver an effective target dose while minimizing traumatic inflammation to the spinal cord (myelitis), bowel (enteritis), bladder (cystitis), and skin (ulceration) and preserve function in the case of liver or kidney. On the basis of conservative α/β ratio predictions, specific recommendations regarding dose constraints can be found in Table 17.2.
GYNECOLOGIC SBRT PLATFORMS
As of now, there are no gynecologic SBRT platform selection criteria restrictions. The most common platforms that offer engineered features for gynecologic SBRT are delineated in Table 17.1. Clinical trials studying gynecologic SBRT uniformly used CyberKnife machine technology to remove confounding radiation dose prescriptions and timetable variability (1, 2), but other platforms amenable to gynecologic SBRT have been studied (13, 24).
DOSE FRACTIONATION AND TREATMENT PLANNING
The gynecologic SBRT prescription used in clinical trials was 24 Gy in three 8-Gy consecutive daily fractions. This prescription has been vetted for safety and efficacy (1, 2). A separate review reported a 2-year local control of 100% when prescribing a target EQD2 dose of greater than 60 Gy, with no reported grade 3 or higher bowel/bladder/rectum toxicity if the cumulative equivalent dose in 2 Gy (EQD2) to critical organs was kept below 110 Gy. Others advise that the maximum point dose to the bowel be kept below 55 Gy (25). This report suggested that multiple fractionation schedules are feasible involving 6 to 20 Gy per fraction (26). Additional reports have demonstrated the feasibility of daily reirradiation schedules of 25 Gy in five fractions and 30 Gy in six fractions (15) as well as 36 Gy in three fractions or 12 to 18 Gy in a single fraction (27).
On most SBRT hardware platforms with up-to-date planning software, the 100% prescription isodose line should cover greater than 95% of the target, with acceptable deviation being a +7% hot-spot dose heterogeneity in tumor. Fixed-collimator CyberKnife gynecologic SBRT studies used radiation dose prescribed to the 70% isodose line to achieve greater than 95% target coverage. It is important to note that there are no restrictions on SBRT prescriptions and isodose line assignment, and it is very likely that broad SBRT prescription variation exists in current practice.
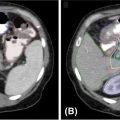
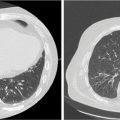
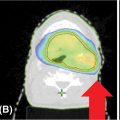
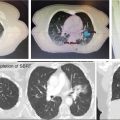
Each SBRT treatment planning system uses proprietary software algorithms for radiation dose calculation. Because SBRT platforms have unique degrees of freedom for radiation beam position and have different means of beam collimation (refer to Table 17.2), treatment plans are frequently complex and diverse. In early clinical experience with gynecologic SBRT, it was observed that variation in tissue thickness, intervening low-density tissue (e.g., lung), breathing, heartbeat, digestive motions, or bladder and rectum fills all introduced variation in calculated radiation dose, target coverage, and target dose heterogeneity. Further study of these parameters, as they related to SBRT platform performance, should be done to guide whether gynecologic SBRT–agent combination clinical trials should be limited to a single platform or be all-platform inclusive. Figure 17.1 demonstrates an example of a patient with a nodal recurrence of squamous cell carcinoma of the cervix who underwent SBRT following previous definitive chemoradiation therapy (50.4 Gy in 28 fractions).
TREATMENT WORKFLOW
For any given SBRT treatment session, the treating radiation oncologist should provide direct on-site supervision. In some practices, at least one radiation physicist and up to three radiation therapists attend SBRT treatment delivery. For patient workflow, the first in-room positioning step necessitates alignment of laser-aligned skin tattoos and wearable motion devices (if any). Pretherapy, machine–patient collision checks are done. On the basis of the needs of each SBRT platform’s motion management, images are acquired for internal target marker alignment. Comparison of treatment day with simulation digitally reconstructed radiograph (DRR) images is done for proper pretherapy alignment. The on-site supervising radiation oncologist approves pretherapy alignment images prior to beam-on. During beam-on treatment, SBRT platform robotics or other hardware–software subsystems compensate for target motion and for positional drift, and should be periodically checked by the treatment team for proper alignment.
275
FIGURE 17.1 The CT (A) and FDG-PET (B) scan of a 50-year-old female with a nodal recurrence of squamous cell carcinoma of the cervix following definitive chemoradiation therapy at a dose of 50.4 Gy in 28 fractions. Shown is a 13-field static SBRT plan (C) targeting the PTV with a dose of 30 Gy in five fractions (D).
FDG-PET, 18F-fluorodeoxyglucose–positron emission tomography; PTV, planning target volume; SBRT, stereotactic body radiation therapy.
OUTCOMES
A CyberKnife SBRT monotherapy phase II trial (NCT01079832) recruited 50 women with one or four or less thoracic or abdominopelvic gynecologic metastases. These women underwent three consecutive daily 8-Gy SBRT dose fractions (1). Strict two-step SBRT dose constraints were used during planning (Table 17.1). On trial, gynecologic SBRT led to attributable grade 2 or higher fatigue (16%), nausea (8%), and diarrhea (4%). Also on trial, one (2%) woman had grade 4 hyperbilirubinemia when gynecologic SBRT was used for a 300-cm3 intrahepatic metastasis. The overall target response rate for gynecologic SBRT was 96% (48 of 50 patients). There were no (0%) SBRT-targeted sites of disease progression. On trial, the overall disease progression rate was 62% (31 of 50 patients), with a median time to elsewhere disease recurrence of 5 months (range, 1–16 months).
A phase I SBRT–chemotherapy combination trial (NCT01652794) recruited 12 women with one or four or less chemorefractory abdominopelvic metastases (2). Dose-escalated carboplatin (area-under-the-curve [AUC] 2 or 4) plus gemcitabine (600 or 800 mg/m2) was given on day 1 (1). CyberKnife SBRT (8 Gy × 3 consecutive daily fractions) was given on days 2 to 4. Carboplatin AUC 4 and gemcitabine 600 mg/m2 in combination with gynecologic SBRT were identified as the maximum tolerated drug doses. On trial, the gynecologic SBRT–chemotherapy combination led to attributable neutropenia (42%), fatigue (25%), thrombocytopenia (25%), nausea (16%), and diarrhea (16%). One (8%) woman developed a grade 3 rectovaginal fistula 16 months after combination treatment. There were no (0%) SBRT-targeted sites of disease progression. Elsewhere, disease recurrences occurred in 75% (nine of 12) of women, with six of the nine women having undergone additional posttrial chemotherapy or hormonal therapy. Median time to disease recurrence was 2 months (range, 1–7 months).
Other clinical experience for gynecologic SBRT in metastatic or previously-irradiated gynecologic tumors remains limited. One study (6) used robotic SBRT in 30 women having isolated para-aortic lymph node uterine (n = 2) or cervical (n = 28) cancer metastases. A nonuniform SBRT prescription ranging between 33 and 45 Gy in three equally divided treatments produced a 96% overall response rate. A 4-year actuarial control rate of 67%, progression-free survival rate of 45%, and 32-month median time to disease progression were reported in this study. Adverse events attributed to SBRT in this study listed five (17%) occasions of grade 3 or higher hematological toxicity. An American CyberKnife study (7) and an Italian conventional linac study (15) found para-aortic lymph node control of 79% and 84%, respectively. SBRT-related adverse events were uncommon and reversible in these two studies.
276QUALITY OF LIFE
Limited data exist regarding patient-reported quality of life specifically following gynecologic SBRT. The rate of grade 3 or higher toxicities to organs at risk is exceedingly low. In the authors’ experience, when planned appropriately, less than 1% of gynecologic SBRT patients develop symptoms such as diarrhea/nausea/fatigue that are not short-lived, self-resolving, or responsive to medication.
TREATMENT SAFETY AND FOLLOW-UP
In the largest clinical trial experience (1), erythema, epilation (loss of hair), or desquamation adverse events were infrequent. Gynecologic SBRT irradiation of the vaginal mucosa leading to discomfort because of vaginal desquamation (10%) was relieved by bacteria-containing plain yogurt, applied topically to the vaginal mucosa by hand or by a vaginal dilator. Grade 3 or higher bladder cystitis (2%) or bowel frequency (2%) within the first 7 days posttherapy was rare. Obstructed bowel or intestinovaginal fistulas (3%) or vesicovaginal fistulas (2%) were also rare. Bone, nerve, and muscle were not often injured (<1%).
Women treated by SBRT should be followed daily on therapy, 4 weeks posttherapy, and 3 to 6 months afterward for care of any adverse events. In some practices, posttherapy CT-based imaging begins at 8 weeks posttherapy.
CONCLUSIONS
Gynecologic SBRT clinical trials have been few and far between despite clinical interest in the technique. It should be appreciated that women with persistent or recurrent metastatic gynecologic tumors still have unmet treatment needs for disease control, and treatments that combine gynecologic SBRT for measurable disease and novel anticancer agents for occult disease remain appealing. New initiatives at the National Cancer Institute bring forth opportunities to study the gynecologic SBRT technique in combination with novel anticancer drugs.
SUMMARY OF DOSE CONSTRAINTS (TABLE 17.2)

REFERENCES