Fig. 11.1
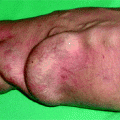
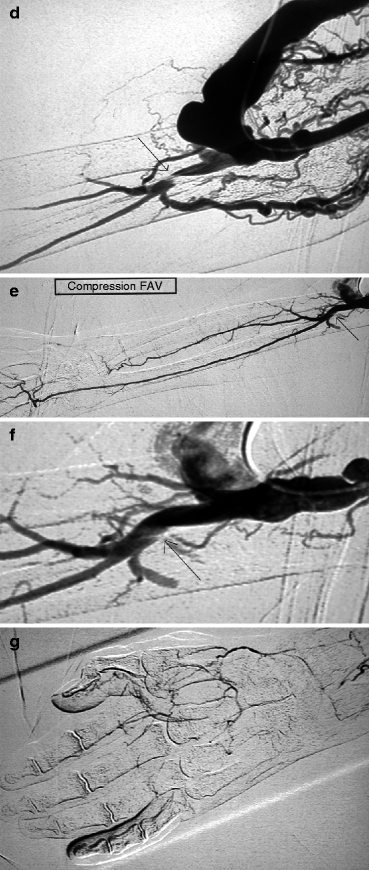
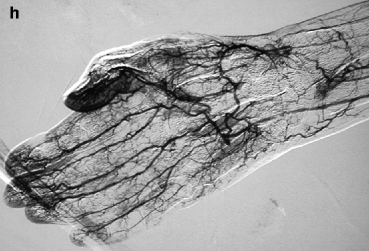
(a, b) This patient was dialyzed for many years via a brachial–cephalic fistula after failure of a radial–cephalic fistula. A venous stenosis in the middle third of the upper arm has been deliberately underdilated in the past and has remained stable for many years after placement of an undersized covered stent. Arteriography performed after she presented initially with intermittent hand pain (stage 2 distal ischemia) which later became constant (stage 3) was through a retrograde puncture of the vein and catheterization of the brachial artery via the anastomosis. Pre-procedure duplex ultrasound findings had given a volume flow of 1.3 L/min, but no information was available on the digital pressures or digital–brachial index (DBI). The entire brachial artery flow goes to the arterialized vein. Flow to the distal brachial artery segment is maintained by collaterals. (c, d) These two images centered on the elbow and forearm showed thrombosis of the radial artery in the forearm along with retrograde distal radial artery flow at the wrist from the palmar arches. There was what looked like a stenosis of the distal brachial artery (arrow) but which was in fact a cutoff in distal opacification as result of diversion of all proximal brachial artery flow into the fistula. Distal opacification was resumed by the first collateral runoff. (e, f) The pseudo-stenosis disappeared (arrow) on subsequent frames taken with the fistula manually occluded. (g) There is minimal opacification of the digital arteries when the fistula is not compressed. (h) Digital arteriography performed with the fistula compressed showed that all the digital arteries were patent and functioning, making the diagnosis of ischemia from distal hypoperfusion very likely. The patient’s pain disappeared after DRIL was performed with no recurrence of symptoms at 2 years’ follow-up
Symptomatic chronic DHIS arises and worsens once distal arterial flow fails to meet the basic metabolic needs of the tissues in the hand. This explains why a normal or hyper flow AVF may be associated with distal ischemia in spite of apparently normal arteries, while a low flow AVF may trigger the same in the presence of diseased arteries or inadequate arterial collateralization (Fig. 11.2a, b).
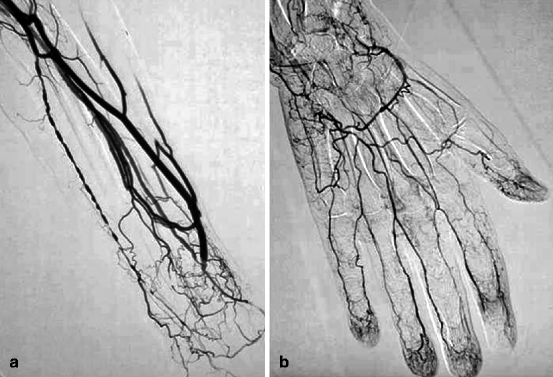

Fig. 11.2
(a) This diabetic patient developed an ulcer in the pulp space of his right index finger a few weeks after creation of this left radial–cephalic fistula. Arteriography pinpointed the cause of low access flow as being due to a radial artery stenosis above the anastomosis and multiple ulnar artery stenoses. (b) A subsequent frame taken with the fistula manually compressed showed persistent absence of right index finger pulp space opacification. In view of the low access flow and technically difficult task of dilating the long segment of ulnar artery stenosis, this case of distal ischemia was treated by fistula ligation
In general, the risk of distal ischemia after dialysis access creation does not only increase in the presence of arterial lesions but is also commensurate with the degree of chronic venous hypertension. Not much is discussed about the latter in the literature.
Despite our current knowledge on DHIS, it is unfortunately still impossible to predict prior to access creation who will develop distal ischemia as it is equally difficult to predict with certainty the enlargement of the feeding artery and arterialized vein and development of arterial collaterals, especially those located between the axillary and forearm arteries in upper arm accesses. Statistically speaking, upper arm AVFs are much more likely to produce DHIS than forearm AVFs [5]. Patients prone to atherosclerosis (diabetics, smokers or ex-smokers, hypertensives, elderly, and high dialysis vintage) and those with known arterial lesions fall in the high-risk category for developing DHIS. Invariably forearm AVFs are associated with severe distal ischemia only in the presence of lesions in the adjacent arteries whether these are found in the ulnar artery in the case of radial–cephalic AVFs, radial artery in the case of ulnar–basilic AVFs, or in the palmar arches.
11.2 An Atypical Form of Acute Ischemia: Ischemic Monomelic Neuropathy (IMN)
Excruciating ipsilateral hand pain associated with motor weakness and sensory loss of sudden onset minutes to hours after access creation is a surgical emergency and should prompt the surgeon to immediately ligate the AVF before there is irreversible motor deficit [6]. This acute clinical syndrome known as ischemic monomelic neuropathy (IMN) is rare and predominantly occurs immediately after creation of an upper arm access in diabetics. The likely pathogenesis is circulatory insufficiency of the vasa nervorum (microvessels perfusing the neurons). The ensuing nerve damage or ischemia is rapidly progressive and can involve all the three major nerves (median, ulnar, and radial) in the forearm. The other tissues do not develop any ischemia as can be expected from the totally normal arterial hemodynamics (i.e., pulse, flow, and velocity). Time should therefore not be wasted in organizing angiography as any minute that lapses without the AVF being ligated produces more terrifying symptoms. The interventional radiologist, in this instance, should never be called in to preoperatively explore possible etiologies for the symptoms. Rapid ligation of the AVF by experienced surgeons aware of this dramatic complication does not always prevent development of long-term debilitating sensorimotor sequelae. From a medicolegal standpoint, vascular surgeons should warn diabetic patients and their nephrologists of this disturbing though rare complication whenever an upper arm access is being planned.
11.3 Chronic Ischemia
11.3.1 Diagnosis
A classification or severity scoring ranging from a scale of 1–4 has been proposed for chronic ischemia and is useful in management planning [7]. Stage 1 denotes a pale, dusky, numb, or cold hand in the absence of pain; stage 2 hand pain, sometimes excruciating and experienced on activity or during dialysis; stage 3 hand pain at rest; and stage 4 presence of ulcers, tissue necrosis, or gangrene.
Cases at stage 1 are usually managed conservatively and carefully observed for progression of symptoms. At stage 2, some form of access imaging, usually in the form of duplex ultrasonography, is mandated to rule out any central arterial stenosis that can easily be dilated. At stages 3 and 4, a diagnosis should be thoroughly and rapidly looked for and intervention urgently instituted.
Based on retrospective observational studies, the risk of DHIS in forearm AVFs is less than 2 %, whereas in upper arm accesses, it can be as high as 28 % [8]. Series from the USA nonetheless describe only DHIS associated with either native or prosthetic upper arm accesses. These figures can only increase with time given that an increasing number of elderly and diabetics are now initiating dialysis. The onset of symptoms occurs much earlier in upper arm than in forearm accesses. In the latter, symptoms may appear years later as a result of hyper flow and increased steal or progressive deterioration of the distal arterial network.
Surprisingly or shockingly, the early recognition and timely referral of DHIS by nephrologists and dialysis nurses are often delayed. Such lapses arise because much focus is placed on the needling segments of fistulas and not on the entire access circuit, which includes the hand. The patients’ timid complaints of hand pain are often not taken in their full weight. The degree of pain experienced may also be dampened by diabetic or uremic neuropathy and therefore poorly reflects the true extent of tissue damage. These factors often make dialysis staff more disinclined on making early referrals, an inaction that can be costly and potentially lead to the loss of the access or the digits. A lack of awareness and adequate training and empowerment of dialysis staff in vascular access is a major challenge worldwide. The severity of ischemia is sometimes underestimated even by surgeons and radiologists not well versed in the management of dialysis accesses.
Consequently, a number of cases are intervened upon tardively, at a point when the clinical situation has become much too desperate. It is not uncommon for the diagnosis of DHIS to be entertained for the first time by sonographers or radiologists evaluating the accesses for a different clinical indication (Fig. 7.1). The rule of thumb for staff working with dialysis patients, be they medical or allied, should therefore be that any complaint of hand pain or the presence of poorly healing digital lesions on the same side of the access should be considered as due to DHIS until proven otherwise. This should then be corroborated by some form of imaging, the simplest being duplex ultrasonography in tandem with arterial blood flow and digital pressure measurements. In practice, angiography however remains the default imaging modality, as not all centers are manned by vascular sonographers, radiologist, or angiologists (in the case of France) well experienced in adequately evaluating accesses and the upper limb arterial supply by duplex ultrasonography for DHIS.
11.3.2 Noninvasive Work-Up
Duplex ultrasonography, an essential noninvasive tool, must involve a detailed study of the entire upper extremity arterial architecture, with mandatory calculation of access flow usually at the level of the brachial artery [9]. When performed by experienced operators, it allows the detection of stenoses from the subclavian to the distal arteries in the hand, in addition to an assessment of arterial blood flow direction, especially that of the artery distal to the anastomosis. The structural and functional quality of the palmar arches can equally be studied. Duplex ultrasonography is also useful in identifying stenoses or surrogate markers of venous hypertension (collaterals) in the arterialized vein from the anastomosis to the central veins, though it is less sensitive at the latter.
The measurement of digital pressures by photoplethysmography, digital–brachial index (DBI), and digital pulse oximetry are critical components of DHIS work-up but are not available in all centers [7, 9, 10]. Any drop in arterial hemodynamic indices (digital pressure or DBI) denotes distal arterial insufficiency. As in lower limb arteriopathy, stages 3 and 4 DHIS are associated with digital pressures below 50 mmHg and/or DBI below 0.6. These hemodynamic indices however have high sensitivity but low specificity, implying they are present in almost all true cases of DHIS as well as in asymptomatic cases.
As a true test of steal, digital pressures and DBI must improve significantly or even normalize on access occlusion. They therefore play a role in differentiating DHIS from other pathologically distinct conditions like carpal tunnel syndrome, calciphylaxis, destructive arthropathies, localized infections, and neuroalgodystrophies which may have similar presentations. Calciphylaxis is a rare systemic arteriopathy related to bone mineral disease (calcium, phosphate, and parathyroid hormone imbalance) and occurs in 1–4 % of ESRD patients. The underlining pathology is medial calcification of medium- to small-sized arteries, leading to peripheral or central tissue ischemia [11].
Pulp space oximetry is another diagnostic tool used sparingly, but its accuracy is not well known given the limited publications on it.
The question then arises as to whether duplex ultrasonography is sufficient enough as first-line investigation for DHIS or whether a systematic angiography should be performed instead. There is no clear answer to this question. However, two prerequisites need to be satisfied before either one of them are considered. First, the ultrasound operator needs to be competent in examining accesses other than just being able to calculate blood volume flow. Second, arteriography should be thorough and detailed enough to provide all the information required to assist the multidisciplinary team, notably the surgeon, often hesitant and imprecise on the appropriate approach to take on this delicate access complication unless thorough and accurate hemodynamic data are available.
In addition, the diagnostic approach should at best be able to offer a platform for same-sitting treatment, especially in the case of proximal arterial stenoses.
The use of magnetic resonance angiography (MRA) as a diagnostic tool prior to iodinated contrast arteriography is controversial and strongly discouraged except under very extreme circumstances and minimum gadolinium contrast agent must be dosed, given the risk of nephrogenic systemic fibrosis in ESRD patients. Indeed the advantage of arteriography over MRA is that it provides better quality imaging and allows concomitant therapeutic intervention on arterial stenoses. CT scan plays no role in the evaluation of DHIS given it requires a big-sized vein, usually at the antecubital fossa, for contrast injection and hence has both a negative impact on venous capital and offers no opportunity for intervention.
11.4 Arteriography
11.4.1 Technique
Arteriography is the most crucial component of DHIS work-up and must include imaging of the entire upper extremity arterial circulation ipsilateral to the access, from the ostium of the subclavian artery to the digital arteries. Runs performed with the arterialized vein compressed or occluded are often necessary to opacify the digital arteries (Fig. 11.1g, h). A nonionic hypo-osmolar iodinated contrast agent, for instance Visipaque®, is preferred as it does not induce a burning painful sensation on injection into the small digital arteries (diluting it by 50 % further makes contrast injection even less painful), which would otherwise cause patient discomfort and restlessness. The arm should remain still throughout arteriography, which is not always possible in the elderly and very sensitive patients.
Access to the arterial system depends on the type of vascular access under study and whether the brachial artery is easily palpable. It is generally recommended that the arterialized vein or graft of upper arm accesses be cannulated retrogradely. A 4-F catheter is then pushed across the anastomosis into the proximal brachial artery right up to the aortic arch to facilitate opacification of the ostium of the subclavian artery.
In the case of forearm AVFs, a venous retrograde approach presents two challenges. First, negotiating a guidewire and a catheter into the proximal radial artery across a smaller anastomosis which is often acutely angled can be laborious not to mention impossible. Second, passage of a catheter into the radial artery can be the source of major spasms locally that can create false impressions and lead to misinterpretation of both AVF and hand opacification. Retrograde cannulation of the brachial artery at the elbow with an 18-G cannula is therefore the preferred approach though this may in turn induce spasm at the site of puncture. It is best to replace the cannula over a straight hydrophilic guidewire with the dilator of a 4-F introducer sheath, which is less likely to kink or be accidentally removed. Retrograde cannulation of the brachial artery allows opacification of the entire arterial architecture of the upper limb distal to the subclavian artery ostium through reflux of 30–40 mL of contrast by pulse injection at 15 mL/s (Fig. 11.3a–d). A larger volume of contrast may be required for better opacification of the arterial supply of right-sided accesses in view of the larger caliber of the arterial brachiocephalic trunk. An additional run with the brachial artery or fistula occluded at the elbow by manual compression must be performed when the reflux of iodine does not provide sufficient opacification of the subclavian artery. As a last resort, a 4-F catheter can be pushed into the aortic arch.
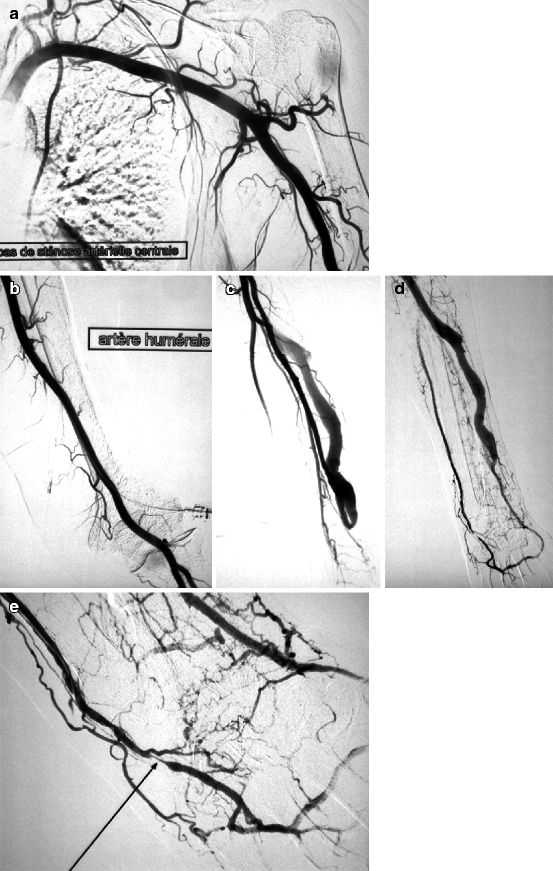
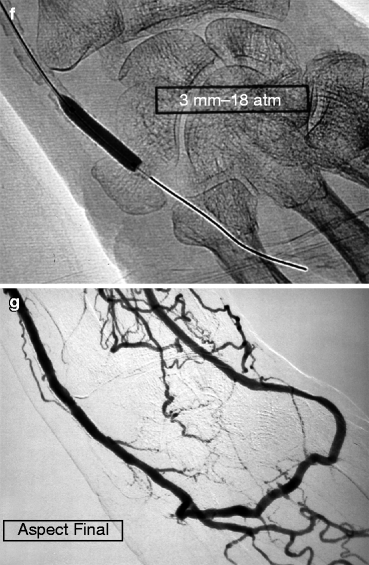


Fig. 11.3
(a–d) This patient dialyzed via this left radial–cephalic fistula for some months without any incident suddenly developed digital ischemia. Access flow was 600 mL/min, and an ulnar artery stenosis was demonstrated on duplex ultrasound. Digital–brachial index was 0.5. Arteriography performed by retrograde cannulation of the brachial artery ruled out any proximal stenosis but confirmed the ulnar artery stenosis, shown here also as delayed opacification of the ulnar artery compared to the interosseous artery (“pas de stenose arterielle central” means “no central artery stenosis”, “artere humerale” means “brachial artery”). (e–g) The ulnar artery stenosis was selectively catheterized from an antegrade puncture of the brachial artery at the elbow using a 5-F introducer. The ulnar artery stenosis (arrow) was crossed using a 0.014-in. guidewire (Spartacore®) and successfully dilated with a 3-mm coronary angioplasty balloon
Brachial artery cannulation is relatively contraindicated in patients on oral anticoagulants like coumadin and warfarin when the INR is above 2. Retrograde cannulation of the distal radial artery impairs a true appreciation of distal arterial flow and renders contrast reflux into the subclavian artery impossible.
Catheterization of the subclavian artery must be performed through cannulation of the femoral artery in the case of a poorly palpable brachial pulse. Arteriography in this case invariably shows a severe stenosis anywhere between the aortic arch and brachial artery above the elbow, which can be dilated in the same setting. A complete arterial occlusion is occasionally encountered. Another indication for femoral artery cannulation is the presence of severe scars or other lesions on the skin overlying the site of brachial artery puncture.
It is a fundamental principle that for every upper limb arteriography, the entire axillary artery and its collaterals are well captured. This means that sufficient contrast must be injected and a long enough time allowed for image acquisition so that opacification of the post-anastomotic arteries, especially those associated with upper arm accesses, is optimized and a high brachial artery bifurcation is not missed out.
11.4.2 Principles of Reading Arteriograms
The interpretation of arteriograms in DHIS should be geared toward corroborating the noninvasive but exhaustive clinical information already provided by digital pressure and DBI measurements (low digital pressure and DBI < 0.6) and duplex ultrasonography. Unfortunately, arteriography is often the first investigation requested by some nephrologists, because of center-specific particularities like lack of reliable duplex ultrasound expertise.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree