6
Head and Neck
Albert Tiong and June Corry
INTRODUCTION
Head and neck cancers encompass a wide variety of anatomic subsites and histologies, though most commonly they are squamous cell carcinomas arising from the oral cavity, pharynx, larynx, and skin. These tumors can cause significant distress for patients with symptoms including: pain, bleeding, obstructed breathing, cranial nerve palsies, and impaired speech and swallowing. Where cure is not possible, radiation therapy (RT) is an effective means to palliate many of these symptoms. Despite treatment, however, many patients will still die of their primary tumor, rather than from metastasis (unlike many other cancers). It is therefore imperative to control the locoregional disease and symptoms and also to ensure that patients do not spend the remaining part of their limited life spans recovering from treatment-related toxicities; for instance, a full course of radical RT to the head and neck region can take an average of 4 to 6 weeks to recover from acute toxicities.1
A head and neck cancer patient may be considered palliative because of the following reasons:
![]() Extent of disease where primary surgery and/or (chemo)RT is unlikely to cure the disease
Extent of disease where primary surgery and/or (chemo)RT is unlikely to cure the disease
![]() Poor performance status
Poor performance status
![]() Comorbidities, which will preclude radical treatment and/or significantly reduce the life-expectancy of the patient
Comorbidities, which will preclude radical treatment and/or significantly reduce the life-expectancy of the patient
![]() Presence of distant metastatic disease
Presence of distant metastatic disease
The decision to treat a patient with palliative versus curative intent can be difficult as both the disease and treatment-related toxicity produce significant symptoms. There is no simple, universally validated algorithm to predict the survival of these patients. Figure 6.1 provides an algorithm for decision making regarding radical versus palliative treatment. There are a number of prognostic factors that should be taken into account when making these decisions.

FIGURE 6.1 Algorithm for selecting patients with head and neck squamous cell carcinoma for radical treatment. ECOG, Eastern Cooperative Oncology Group.
Performance Status, Comorbidities, and Age
Performance status reflects the interaction between a patient’s age, medical comorbidities, and tumor burden. While age is an adverse predictor of cause-specific survival, the effect appears to be modest.2 Of greater importance is the presence of comorbidities, performance status of patients, and the impact of age on organ function and reserve, which have significant impact on the overall survival of patients.3
In patients who have become deconditioned as a result of tumors, several strategies may be employed in an attempt to improve their performance statuses. Improving a patient’s nutritional status and pain medication regimen may facilitate curative treatment. Nutritional status can be addressed by appetite stimulants, enhancing protein intake, and addressing issues that interfere with oral intake. Placement of a nasogastric or percutaneous enterostomy feeding tube may be required. Induction chemotherapy may have the same effect by reducing the tumor burden. Although these strategies are yet to be validated in prospective trials, they are not uncommonly used in clinical practice.
Anatomic Subsite and Biological Markers
The potential curability of a tumor is highly influenced by the subsite of disease and presence of biological markers. For instance, undifferentiated nasopharyngeal carcinomas are known to be highly sensitive to both RT and chemotherapy and despite advanced locoregional disease, cure is still achievable.4 Similarly, human papilloma virus (HPV) associated oropharyngeal carcinomas in nonsmokers can have high cure rates despite locally advanced disease.5 Subsites such as the oral cavity and hypopharynx have traditionally been much more difficult to cure in very advanced disease; careful consideration of other prognostic signs should occur before considering radical treatment.
Stage and Volume of Disease
In general, the higher the stage of disease, the lower the locoregional control rates. The T- and N-stage, however, may not tell the whole story. The volume of disease is independently predictive of local control on multivariate analysis.6 Patients with undifferentiated nasopharyngeal carcinomas and HPV-positive oropharyngeal carcinomas can have a high complete response rate to treatment despite high-volume disease.
Histology
Although squamous cell carcinoma is the most common histology in the head and neck, there are other rare entities that occur in this region. The selection of intensity of treatment is dictated by the natural time course of disease. For instance, adenoid cystic carcinomas have a high propensity to recur distantly but can have protracted courses with survival measured in years. It is therefore important not to undertreat the local disease sites and associated perineural areas of risk.7
Distant Metastasis
The presence of distant metastasis has traditionally meant that patients were considered incurable from their disease. This paradigm may be changing for selected patients. In patients with undifferentiated nasopharyngeal carcinoma and a limited number of distant metastasis, aggressive treatment can achieve very durable (more than 3 years) progression-free survival.8–10 This may also apply to HPV-positive patients with distant metastasis.11 In other subsites, there is currently not enough evidence to treat these patients aggressively.
Despite known prognostic factors, the decision to recommend radical treatment may be difficult. Performance status, stage, and volume of disease have the greatest impact on this treatment decision. Algorithms that incorporate clinical and disease factors to predict the survival of head and neck cancer patients after treatment are listed in Table 6.1. More general prognostic tools can be found in Chapter 2 and Appendix B. Ultimately, predicting whether a patient will tolerate radical treatment and achieve disease control depends on the clinical experience and acumen of the physician.
TABLE 6.1 Some Published Algorithms for Predicting the Survival of Patients with Head and Neck Cancers
Subsite | Study | Comment |
Oropharynx | Velazquez et al.31 | Prediction of 2- and 5-year survival of patients who were treated with 70 Gy/35 fractions of (chemo)RT based on Hb, gender, smoking, TN, p16 status, and comorbidities. |
Oropharynx | Ang et al.5 | Prediction of 3-year OS (low, intermediate, or high) of patients with oropharynx cancers based on p16 status, TN, and smoking pack-year. Only patients with good PS (Zubrod 0 or 1) were included in this study. |
Oropharynx | Rietbergen et al.32 | Prediction of 3-year OS (low, intermediate, or high) of patients with oropharynx cancer based on p16 status, TN, smoking pack-year, and ACE-27 comorbidity index. |
Larynx | Egelmeer et al.33 | Nomogram to predict LC and OS of patients with glottic cancers who are treated with RT alone. Factors that were predictive were age, gender, TN, glottic versus non-glottic, Hb, and RT dose (in 2 Gy equivalent). Comorbidity data were not collected in this population and patients treated with chemotherapy were excluded. |
Multiple mucosal subsites | Datema et al.3 | Predictive model for survival based on inputs of age, gender, TNM, prior malignancy, ACE-27, and tumor subsite. Unfortunately, the interface is not freely available. |
Multiple mucosal subsites | Wang et al.34 | Model for predicting OS if patient survives treatment from cancer at time points from completion of treatment. Data are based on SEER and hence crude (variables entered include race, site, sex, non-TNM stage, and grade). |
ACE-27, adult comorbidity evaluation-27; Hb, hemoglobin; LC, local control; OS, overall survival; PS, performance status; RT, radiation therapy; SEER, Surveillance, Epidemiology, and End Results Program; TNM, tumor, nodal, and distant metastasis staging.
Multidisciplinary discussion that includes radiation oncologists, medical oncologists, surgeons, speech pathologists, dieticians, nurses, and social workers can facilitate treatment discussions. It is worthwhile to clarify the intent of treatment; whether it is for cure or palliation, how high or low the probability of cure, and whether the goal is to palliate current or imminent symptoms versus prolong disease control and possibly survival.
RT is an effective treatment for locoregional symptoms and produces a higher response rate when compared with chemotherapy.12–26 Therefore, where local or regional disease is the predominant cause of patients’ symptoms, RT should be used. Chemotherapy is the initial treatment approach when distant metastases predominate or if further radiation is limited because of prior treatment. Embarking on retreatment with RT in the head and neck should be made after careful consideration of the potential side effects versus the benefits. Median survival with full-dose irradiation (60 Gy) entails significant treatment-related morbidity (8%–10% grade 4 reactions) with somewhat limited survival (median survival 8–12 months).28,29
In the palliative setting, there are multiple trials using a wide range of different dose fractionation schedules. Table 6.2 summaries the current literature regarding RT in head and neck cancers. These trials show that RT is an effective means to palliate symptoms. As an example, palliative RT may achieve the following improvements:
![]() Pain (56%–67%)
Pain (56%–67%)
![]() Dysphagia (33%–53%)
Dysphagia (33%–53%)
![]() Voice quality (31%–57%)
Voice quality (31%–57%)
In the palliative context, the best RT regimen is an unanswered question. Unfortunately, trials in the palliative context are extremely heterogeneous with regard to clinical factors such as patient’s age, performance status, and stage of disease. Hence, direct comparison of different regimens is difficult. What is clear, however, is that higher doses of radiation produce more side effects, with up to 65% grade 3 mucositis.14 This may not be justified in a patient who will survive 6 to 9 months, as a considerable amount of time will be spent recovering from acute toxicities.
In those with very poor prognoses (i.e., <3 months survival), single fraction regimens (8 Gy), are preferred. If needed, the dose can be repeated twice more at weekly or so intervals. Single fractions are often used for bleeding or pain but are unlikely to produce durable local control. For patients with a predicted survival of 3 to 9 months we recommend the QUAD shot (14 Gy in 4 fractions, twice per day over 2 days or daily over 4 consecutive days, and repeated monthly for a total of three times) or a similar hypofractionated regimen. This regimen demonstrated effective palliation of symptoms with minimal toxicity (10% grade 2 mucositis and no grade 3 toxicity; Figure 6.2).12 For patients with a predicted survival of more than 9 months, higher doses (EQ2 for late toxicity of 55 Gy equivalent or more) may be justified as trials suggest a better tumor response (e.g., 45% complete response rate with 50 Gy in 16 fractions compared with no complete responses in the QUAD shot study). The addition of concurrent chemotherapy in the palliative context has been explored but it is uncertain whether this provides additional benefit.30
TABLE 6.2 Studies on palliative RT for HNSCC




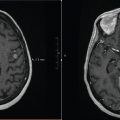
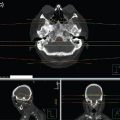
FIGURE 6.2 Simple conformal technique to deliver palliative radiation therapy (RT; QUAD shot) for a T1N3 SCC right tonsil in an 82-year-old male with multiple comorbidities, ECOG 3. (a) Tonsil level, (b) lower neck level, (c) coronal view, (d) sagittal view.
TREATMENT PLANNING
Immobilization
Patients are positioned supine with a thermoplastic mask fixed at five points in most instances. This is to ensure that treatment is reproducible and that planning target volume (PTV) margins can be kept minimal (5–10 mm) to minimize acute toxicities.
CT scanning
Patients are CT scanned with a standard image acquisition (3 mm slices). For lower dose treatments (e.g., QUAD shot) we do not use contrast. In most instances the tumor can be seen well enough on diagnostic imaging and slightly larger margins may be used to account for delineation uncertainties. Where higher doses are used, IV contrast can be justified to assist in delineation. Where resources are limited, two-dimensional simulation with field border markings is used instead.
Target Volume Delineation
Gross tumor (primary and nodes likely to be symptomatic in the lifetime of the patient) should be delineated. When available, imaging modalities acquired in the work-up of the patient should be used (either side by side or with fusion).
Prophylactic nodal irradiation (PNI) in general is not warranted. It may be considered in situations where high-dose palliation is used, where there is a reasonable chance of complete or near complete response in the gross disease. In this setting, an untreated node may progress and become symptomatic, and reirradiation might be difficult. We recommend that treatment volumes cover a single echelon of lymph nodes on either side of the gross disease. PNI needs to be considered in the context of potential toxicity. Where the potential side effects are low (e.g., lower neck field), then it may be justified. If toxicity is likely to be increased significantly (e.g., treating bilaterally through midline structures), then the benefit of PNI should be weighed against the additional morbidity. Irradiating gross disease will produce the most clinical benefit.
In general, the PTV should be a 5 to 10 mm expansion on the gross tumor volume (GTV). Whether a clinical target volume (CTV) should be used in the palliative context to cover potential microscopic spread is debatable. Figure 6.2 shows an example of a typical volume and dosimetric coverage for a QUAD shot.
Normal Organ Tolerances
Given the limited life spans of patients with metastatic head and neck cancer, late effects are unlikely to cause problems. However, with palliative regimens, dose constraints are used for organs at risk where late effects could have potentially devastating consequences. For example, with the QUAD shot regimen, we limit the maximum point dose to the spinal cord to 28 Gy, which is easily achievable in most instances. Where the tumor lies close to critical structures, the underdosing of the GTV needs to be weighed against the clinical likelihood of late toxicity. Late toxicity with most palliative regimens is unlikely to occur in patients with life expectancies of less than 6 months. Other normal structures to be mindful of include the oral cavity and midline structures (larynx, pharynx) and major salivary glands. The dose to these structures should be minimized to reduce acute toxicity, which can affect quality of life.
Dose Solutions
In resource-limited environments, the simplest field arrangement should be used (e.g., parallel opposed or three-field plans). This also has the advantage of limiting the time for which the patient has to be on the treatment couch. Where resources are available, intensity-modulated radiation therapy (IMRT) has the potential to spare some toxicity.26 In our experience, a QUAD shot in most instances produces minimal mucositis with conformal treatment and IMRT would not add a significant benefit. If higher doses are prescribed, IMRT is much more likely to add a clinical benefit by sparing organs such as the parotid glands and midline structures.
Dose-Fractionation
Based on published literature and institutional experience we suggest the following as a guide (though there are many other dose-fractionation schedules that are acceptable):
![]() Prognosis less than 3 months: best supportive care or short courses that may help with bleeding or pain (8 Gy, which may be repeated at day 7 and day 21, total dose 24 Gy).
Prognosis less than 3 months: best supportive care or short courses that may help with bleeding or pain (8 Gy, which may be repeated at day 7 and day 21, total dose 24 Gy).
![]() Prognosis 3 to 9 months: 14 Gy in 4 fractions; two fractions per day over 2 days (at least 6 hours apart) or daily over 4 consecutive days. This may be repeated two times on a monthly basis. This treatment can improve a patient’s current symptoms or delay/prevent symptoms that may happen in the future.
Prognosis 3 to 9 months: 14 Gy in 4 fractions; two fractions per day over 2 days (at least 6 hours apart) or daily over 4 consecutive days. This may be repeated two times on a monthly basis. This treatment can improve a patient’s current symptoms or delay/prevent symptoms that may happen in the future.
![]() Prognosis more than 9 months: 50 Gy in 20 fractions to palliative symptoms and potentially prolong survival.
Prognosis more than 9 months: 50 Gy in 20 fractions to palliative symptoms and potentially prolong survival.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree