9
Head and Neck Brachytherapy
J. Nicholas Lukens, Kenneth S. Hu, Peter C. Levendag, David N. Teguh, Paul M. Busse, and Louis B. Harrison
Brachytherapy is an important option in the armamentarium of a radiation oncologist treating head and neck squamous cell carcinoma (SCC). It involves the implantation of radioisotopes into tumors to allow high dose conformality, with dose intensification to areas of high-volume disease, and rapid inverse square falloff to the surrounding normal tissue. It can play an integral role in organ- and function-sparing strategies, which can maximize a patient’s quality of life (QOL) functionally, psychologically, and cosmetically.
A BRIEF HISTORY OF HEAD AND NECK BRACHYTHERAPY
Brachytherapy as a mode of cancer treatment is as old as the history of radiotherapy itself, and head and neck tumors were among the first sites to be treated with brachytherapy. Shortly after the discovery of radium in 1898 by Marie and Pierre Curie, its potential clinical applications were explored. The earliest applications of radium were crude devices placed over the skin, primarily for the treatment of nonmalignant skin diseases. The first mention of the application of radium for the treatment of a tumor was in Vienna in 1902, to treat a cancer of the palate and pharynx (1). In 1904, Wickham and Derais used sharpened goose quills to perform intratumoral implantations, utilizing a primitive manual afterloading technique (2). Dr. Robert Abbe, a brachytherapy pioneer in the United States, is credited with the first successful interstitial implant, treating a 17-year-old boy with a destructive giant cell sarcoma of the lower jaw in 1904 (3). Initially, a variety of head and neck sites were treated using rigid radium sources of finite length to deliver low dose rate (LDR) brachytherapy. In 1958, afterloading techniques using flexible iridium-192 (192Ir) wires were introduced, reducing radiation exposure to staff, and renewing interest in brachytherapy. This led to the development of new rules of implantation and dose calculation for interstitial brachytherapy using 192Ir wire sources, the Paris System, which formed the foundation for modern head and neck brachytherapy (4). Briefly, when uniform linear parallel sources are used, these rules result in a reference isodose that covers the treatment target volume. The reference isodose is 85% of the average basal dose, which is defined by the minimum dose between sources along the central plane of the implant. Considerable experience was gained in the treatment of various head and neck sites with LDR interstitial brachytherapy using 192Ir wire sources, including the lip, oral tongue, floor of mouth (FOM), buccal mucosa, oropharynx, nasopharynx, and neck. The data supporting the use of LDR brachytherapy for these individual subsites are discussed in detail in the following pages. Over the past two decades, there has been mounting clinical experience with the use of high dose rate (HDR) and pulsed dose rate (PDR) brachytherapy in the treatment of head and neck sites. As a whole, the results obtained with HDR and PDR appear to be equivalent to those obtained with LDR brachytherapy in terms of local control and complication rates (5). However, the optimal dose and fractionation for various head and neck subsites, when using HDR brachytherapy, have yet to be established through large studies with long follow-up. Recently, the use of brachytherapy has been combined with intensity modulated radiation therapy (IMRT), to yield excellent oncologic and functional results (6).
GENERAL CONSIDERATIONS
Some general considerations that apply to all head and neck subsites are discussed here, followed by a detailed discussion of the evidence basis, indications, and methods for the application of brachytherapy to treat various subsites within the head and neck.
Although there are no phase III prospective randomized double-blind trials comparing brachytherapy with conformal fractionated external beam radiotherapy (EBRT) in any select group of patients with head and neck malignancies, there are many decades of clinical experience supporting the implementation of brachytherapy as part of the optimal treatment strategy for various head and neck sites.
Within the head and neck, brachytherapy alone can be used in the definitive treatment of smaller lesions, or brachytherapy can be used to deliver a boost in combination with EBRT to treat cancers definitively or in the adjuvant setting. In advanced cancers, brachytherapy may be used in various combinations with EBRT, chemotherapy, and surgery. When used as the sole modality, brachytherapy may preserve the option of EBRT for possible future lesions that may develop. In patients previously treated with EBRT, brachytherapy can play a pivotal role in the treatment of recurrent head and neck disease.
PATIENT SELECTION
The appropriate application of brachytherapy begins with the proper selection of patients. Patients with medical comorbidities who have contraindications for surgery may be poor candidates for brachytherapy. In addition, patients with alcohol dependence, major neurologic deficits, poor cardiopulmonary status, memory disorders, and hematologic disorders may also not be ideal candidates. Age alone is not a contraindication. In addition to various medical conditions, patients must also be assessed for their understanding and ability to comply with the necessary inherent radiation precautions and procedures associated with brachytherapy implants, especially for LDR implants. Patients must be able to provide baseline self-care needs during the radiation delivery. This can include tracheostomy care, self-administered feeds through percutaneous endoscopic gastrostomy tube or a nasogastric tube, and a patient-controlled analgesic pump, as indicated. Patients with periods of confusion and disorientation would not be ideal candidates. The potential for alcohol or narcotic withdrawal should be addressed to avoid complications with the delivery of the implant. Patient-related factors associated with severe soft tissue and bone complications after an implant include severe diabetes, liver failure, and compromised arterial status (7).
Evaluation of the patient’s oral–dental hygiene is also necessary, especially with regard to risk of mandibular osteoradionecrosis. Examination by a dentist or an oral surgeon familiar with radiotherapy (RT) is an integral part of the initial evaluation for brachytherapy for a subset of patients. If dental extractions are required, complete healing must be ensured prior to brachytherapy to avoid the risk of osteoradionecrosis. Patients must be able to tolerate a custom-designed mandibular shield in the oral cavity during the radiation delivery as protection against osteoradionecrosis (Figure 9.1) (8). This shield reduces the dose received by the mandible by approximately 50%.

Figure 9.1 A custom-designed dental shield lined with lead. A dental cast is used to make the shield. Courtesy of Louis B. Harrison, MD.
Patient selection also includes an assessment of the appropriateness of a brachytherapy implant regarding the tumor location, size, extent of tumor volume, and organ function. The indications and contraindications by subsite are discussed in detail in the following pages. In general, brachytherapy is contraindicated for lesions that are abutting or invading into a bone because of a higher risk of osteonecrosis. The tumor needs to be accessible and should have a geometry that can be encompassed by a series of catheters or sources. Very large tumors for which it is difficult to assess the extent of infiltration are not ideal for brachytherapy implantation due to the risk of a geographic miss.
GENERAL TYPES OF HEAD AND NECK BRACHYTHERAPY
The most common type of brachytherapy used in the head and neck is interstitial brachytherapy, followed by surface applicator techniques and intracavitary applications. In interstitial brachytherapy, the radioisotope is placed either temporarily or permanently into the tumor site or bed. A permanent interstitial implant may be advantageous when the target volume is irregular and complex, making temporary catheter placement impractical and avoiding situations that result in potential kinking of the catheters. In addition, a permanent implant allows for a higher total radiation dose to be delivered to the target volume. A commonly used radioisotope for permanent implants is iodine-125 (125I), which is characterized by a low average energy (28 keV), with rapid dose falloff to minimize dose to normal structures. This may be advantageous when critical normal structures, such as the spinal cord, are adjacent to the tumor implant.
Temporary interstitial implants are more commonly used for head and neck cancer. This involves loading sources (usually 192Ir) into catheters that have been implanted into the tumor volume. This allows for deliberate and accurate placement of the catheters, and optimization of the implant dosimetry using three-dimensional (3D) planning systems.
Surface applicator techniques have been used successfully to deliver radiation intraoperatively to the exposed tumor bed after a gross total resection, and in the treatment of skin tumors and superficial soft tissue sarcomas of the head and neck region. Intracavitary brachytherapy techniques have been developed for the treatment of nasopharyngeal carcinoma, as well as for tumors of the paranasal sinuses and the nasal vestibule.
COMMON INTERSTITIAL TECHNIQUES FOR HEAD AND NECK BRACHYTHERAPY
Successful brachytherapy requires meticulous placement of radioactive sources in the planned tumor volume. Knowledge regarding the extent of tumor from palpation and inspection of the disease, prior radiologic examination, awareness of adjacent critical structures, prior radiation exposure, and relationship of the tumor to the surrounding structures are critical for optimal and safe placement of the radioactive sources. There are various techniques with many modifications over the years. Currently, most interstitial head and neck implants are based on an afterloading system with computerized treatment planning. Some interstitial techniques are described here.
• Pierquin and Chassagne Guide-Gutter or Hairpin Technique
This approach uses hairpins of various sizes consisting of two parallel branches, which provide rigid guides to facilitate the implantation of iridium wires into the tumor. The guide materials are either twin or single guide gutters made of stainless steel. Once the iridium has been implanted, the gutter guides would then be removed, resulting in predictable implant geometry. This technique is suitable for small- to moderate-volume tumors.
• Plastic Tube Technique of Henschke
This is one of the most popular techniques, with various modifications. Rigid metal hollow guide needles are implanted into the tumor volume, typically free hand, although templates are available. The placement and spacing can be verified by visualization, palpation, ultrasound guidance, or fluoroscopy. Plastic tubes are then threaded through the rigid hollow needles and left in place to cover the target volume, with subsequent removal of the metal needles. The plastic tubes are secured at the level of the skin with metallic buttons. Radioactive sources are then afterloaded into the catheters following dosimetric planning. The various instruments needed to facilitate the implant are pictured in Figure 9.2. Large tumors are effectively treated with this approach. There are several variations of the plastic tube technique, including:
– Through-and-Through Technique
This approach is mainly suited for tumor volumes when both sides are accessible for implantation, including the lip, buccal mucosa, skin and neck nodes. The first step is the identification of the intended implant target volume and the points of entry for the brachytherapy catheters, which are arranged in parallel, approximately 1 cm apart. Using a metal needle or trocar, the skin is pierced at the planned entry site, courses along in the tumor volume, and exits at the marked skin site at the other end of the target volume. Once in place, an afterloading catheter is threaded through the trocar, which is then removed along its original pathway while holding the implanted catheter in place. A metal button and a plastic button are threaded over the exposed ends of the catheter and crimped in place over the skin entry sites. The exposed end of the catheter is then cut off leaving at least several centimeters distal to the metal button. These steps are repeated with parallel catheters until adequate coverage is obtained. Depending on the site, patient’s cormorbidities, and the extent of implant, the procedure can be done under local or general anesthesia.
– Loop Technique
This approach has technical aspects that are similar to the through-and-through technique. However, the catheters are looped, usually over a mucosal surface, and exit adjacent to the catheter entry site. This technique is most commonly used for oral cavity and oropharyngeal sites. Because this is commonly done for tongue implants, the technique is discussed for such an implant. After identification of the planned target volumes, critical adjacent normal structures should be delineated with a surgical marker, including the carotid artery, the facial artery, and the hyoid bone. Once the target volume has been assessed, the overall strategy for implantation should be planned out regarding the number of catheters, orientation, placement, and their entry and exit sites. A curved metal trocar is inserted through the submental region, traversing through the site of disease and exiting the dorsal mucosal surface of the tongue. An afterloading catheter is then threaded through the trocar, and held in place as the metal trocar is removed along its original entrance pathway. A similar insertion is then performed with the metal trocar adjacent to the prior entry point, and exiting out of the tongue approximately 1.0 cm away from the prior exit point. The end of the catheter, which is in the patient’s oral cavity, is then looped back and threaded through the adjacent trocar until the catheter end is appreciated at the other end. While securing the catheter, the trocar is then carefully removed, and the exposed ends of the catheter are secured using metal buttons. Sutures are typically not necessary to secure the position. These steps are repeated, resulting in a set of looped catheters in multiple planes covering the target volume. Typically, each plane is approximately 1 cm from the others (see Case 9.2 on Oral Tongue for an illustration).

Figure 9.2 Various instruments for an interstitial head and neck implant. From left to right: (1) A nylon afterloading flexible catheter, 60 cm long, with an inner wire to prevent kinking of catheter. (2) A curved or a straight stainless steel trochar. (3) Metal buttons that can be crimped to secure the catheters and the sources, with holes on the button that allow suturing to the adjacent skin. (4) Needle driver for crimping metal button. (5) Radiopaque dummy sources spaced 1 cm apart, with different identification schemes to help differentiate between multiple catheters.
– Sealed End Technique
In contrast to the through-and-through technique, the catheters exit through only a single side of the implanted target volume. Although this technique is more commonly utilized outside the head and neck in situations when it is not pragmatic to have the catheters exit through both sides of an implant target volume, it can be used for the placement of interstitial catheters into the neck after neck dissection for recurrent disease. As before, the procedure begins with identification of skin entry points and target volume delineation, which often includes the tumor bed with margin. A metal trocar is percutaneously inserted, and an afterloading catheter is threaded through the trocar. After retraction of the trocar, absorbable sutures are used to tie the catheter in place at various intervals to stabilize its position. This step is then repeated, resulting in a parallel distribution of the catheters over the target volume. Typically, the catheters are approximately 1 cm apart in a single-plane implant, 1.2 cm in a multiplane implant, and 1.5 cm apart from each plane. Each of the catheters would then be secured using metal and half-moon-shaped buttons crimped and sewn into place over the entry point.
• Thread Technique
Radioactive sources are braided onto a suture material. The sutures are then sewn into the target volume in its desired positions. A modification of this technique includes threading the suture seeds into a mesh, which is then secured over the tumor bed.
• Direct Implantation Method
The radioactive sources are directly implanted permanently into the planned target volume. A specialized applicator would be used to facilitate the implant. This requires meticulous planning and radiologic guidance for accurate placement of the seeds to achieve good geometry and dose distribution.
MULTIDISCIPLINARY COORDINATION FOR HEAD AND NECK IMPLANTS
To perform a successful brachytherapy implant, a radiation oncologist needs the coordinated support of an experienced team consisting of an anesthesiologist, head and neck surgeons, plastic surgeons, a dental surgeon, and physicists. The placement of the surgical incision, grafts, drains, tracheostomy, and wound closure techniques need to be meticulously planned and discussed before any surgical procedure to optimize the implant geometry and reduce the risk of wound complications. In patients with temporary implants, it should be ensured that surgical drains and wound dressings are appropriately placed to avoid hindrance to source loading and unloading. In addition, coordination of the wound closure procedure will minimize potential tension, damage, and distortion of the implanted catheters and geometry. In the postoperative period, a well-trained nursing staff is critical to ensure avoidance of wound complications, and for patient education with regard to self-care.
TREATMENT PLANNING, DOSE PRESCRIPTION, AND REPORTING
In the case of temporary interstitial implants, the placement of brachytherapy catheters into the target volume is followed by dosimetric optimization using 3D planning systems to reconstruct the implant geometry, clinical target volume (CTV), and organs at risk. This can be done using orthogonal X-rays, CT, and/or MRI imaging of the implant. Dummy seeds can be placed within the implanted catheters prior to imaging to provide the relative seed position. The use of CT and/or MRI for target volume delineation and dose calculation is highly recommended. This allows for the definition of a gross tumor volume (GTV) and CTV, following the recommendations of International Commission on Radiation Units and Measurements (ICRU) Report 58 for dose prescription and reporting in interstitial brachytherapy (9). Typically, the CTV incorporates sites of potential microscopic disease, and in cases of definitive brachytherapy includes a 0.5 to 1.0 cm safety margin expansion on the GTV (10). Organs at risk, such as the mandible, can be contoured, and dose−volume histograms (DVHs) constructed, allowing for objective reporting and potential correlation with clinical outcomes.
Dose prescription and reporting should be based on the ICRU Report 58, which includes recommendations based on the Paris System (9). In addition to reporting the GTV and CTV, the “Treated Volume” is encompassed by the isodose corresponding to the minimal peripheral dose to the CTV. Additionally, a description of the sources, techniques, time pattern, and prescription dose should be documented. Common quality parameters, such as the dose nonuniformity ratio (DNR), homogeneity index (HI), and uniformity index (UI), should also be recorded. The goal DNR (defined as V150/V100) at the authors’ institution is generally less than 25%.
DOSE RATE CONSIDERATIONS: LDR, HDR, AND PDR
The prescribed dose and dose rate are important considerations, which take into account clinical, anatomic, and radiobiologic considerations. Specific dose recommendations by head and neck subsite are discussed in the following text; here we discuss considerations with regard to dose rate.
Historically, LDR brachytherapy was utilized for head and neck brachytherapy, and considerable clinical data demonstrating its safety and efficacy established LDR as the “gold standard.” The theoretical advantages of LDR brachytherapy include the continuous exposure of cancer cells to radiation, exploiting cell cycle-specific radiosensitivity, and increased repair capacity of normal tissues, thought to result in a lower risk of late toxicity. When using LDR, a dose rate between 0.3 and 0.6 Gy/hr is recommended to reduce the risk of late complications (11).
There are now mature data to support the use of HDR brachytherapy for several head and neck subsites, although generally these series report on a small to moderate number of patients. The advantages of HDR are an enhanced ability to conform the implant dosimetry to the target volume, the decreased risk of radiation exposure to medical personnel, and better dose distribution homogeneity within the target volume, with potential for less normal tissue irradiation. In addition, because of the decreased radiation delivery time, there is less likelihood of organ movement, and a higher likelihood of treating the patient as an outpatient. Because of radiobiologic differences with a high dose per fraction, the introduction of HDR brought concerns regarding the risk of increased late complications. At this time, there are studies with moderate follow-up demonstrating equivalent oncologic outcomes and late toxicity with HDR as compared to LDR brachytherapy for the most common head and neck subsites, including the nasopharynx, oropharynx, lip, oral tongue, and recurrent neck disease. The data are reviewed under each subsite, but generally speaking, doses between 3 and 4 Gy per fraction are recommended (10,12). Twice-daily fractions are often delivered, with an inter-fraction interval of at least 6 hours.
PDR brachytherapy combines the advantages of a remote afterloading technique with the radiobiologic benefits of LDR brachytherapy (13,14). Patients receive more frequent low-dose “pulses” every 1 to 3 hours in an effort to mimic continuous LDR treatment. The optimal dose per pulse and the time interval between pulses remain under debate. Long-term results have been published for the fractionation schedule that most closely mirrors LDR brachytherapy, consisting of pulses of 0.4 to 0.7 Gy every hour, 24 hours per day (14). These results demonstrate comparable results to LDR brachytherapy with regard to local relapse-free survival (RFS) and complications. Other authors have suggested a slightly higher dose per pulse, delivered once every 3 hours, with or without a night break; however, there are no prospective long-term data as of yet to support this approach (12,15).
PATIENT MONITORING AND CATHETER REMOVAL
The patient must be monitored closely during delivery of brachytherapy to ensure that the catheters or applicators as well as the sources remain in satisfactory position for the duration. Patients need to be provided with adequate analgesics and nutritional support through a percutaneous endoscopic gastrostomy (PEG) tube or nasogastric tube. Prophylactic antibiotics are often prescribed to prevent skin or soft tissue infection.
Following completion of brachytherapy, removal of the catheters should be done with the coordination of the head and neck surgical team. Before the removal of the catheters, patients must have intravenous access. Suction, dressing materials, and adequate analgesics are also needed. A possible complication that may occur during the removal of the implanted catheters is arterial hemorrhage, which can be effectively controlled with bi-digital compression. A review of the procedure, including a discussion of possible bleeding, should be clearly discussed with the patient to ensure proper cooperation and safety.
Prior to discharge, expected radiation side effects, including potential mucositis, pain, and decreased nutritional and fluid intake, should be carefully reviewed with the patient. Patients can expect to develop mucositis approximately 1 week after completion of brachytherapy, with symptoms peaking by week 3, and subsiding around week 6. Education about this expected reaction is an important part of patient care, as is ensuring availability of analgesics, mouthwashes, and alimentation. Follow-up should be arranged approximately 2 to 3 weeks following discharge from the hospital.
VARIOUS HEAD AND NECK SITES
Nasopharynx
Cancer of the nasopharynx is primarily treated with RT with concomitant chemotherapy for locoregionally advanced disease (16). The nasopharynx is surrounded by multiple critical structures such as the brainstem, pituitary, optic chiasm, temporal lobes, cochlea, and salivary glands. Treatment of nasopharyngeal tumors can be particularly challenging, especially for those that are locally advanced or recurrent. Local control is paramount because it is one of the most important prognostic factors, and is an independent prognostic indicator of distant metastases, besides T and N stage (17). Tumor recurrences are particularly challenging because the surrounding critical structures have received the upper safe limits of radiation exposure. With the advent of 3D conformal RT, IMRT, and stereotactic radiosurgery, the ability to treat with highly conformal RT is available. However, carefully delivered brachytherapy can provide the most conformal treatment approach because of its steep dose falloff and dose optimization potential. The primary role of brachytherapy in the nasopharynx is as a boost to EBRT in early stage cases, or in the management of locally persistent or recurrent disease.
Evidence Basis of the Practice
There is considerable experience using intracavitary brachytherapy as a boost for the primary treatment of early stage nasopharyngeal carcinoma, or for persistent localized disease. There are also data supporting the use of intracavitary or interstitial brachytherapy in the setting of recurrent disease.
Intracavitary Brachytherapy for Primary or Locally Persistent Nasopharyngeal Carcinoma
Wang reported markedly improved 5-year local control of T1 to T2 lesions treated with 60 to 64 Gy EBRT followed by a 10 to 15 Gy boost using an intracavitary cesium implant, 90% compared to 59% for patients treated to 65 to 70 Gy with EBRT alone (18). Levendag et al developed a flexible nasopharyngeal applicator to deliver a fractionated HDR brachytherapy boost of 11 to 18 Gy at 3 Gy/fraction following 60 to 70 Gy EBRT (19). Their initial report demonstrated high rates of local control relative to nonbrachytherapy patients, with little significant late grade 3 toxicity other than synechiae of the nasal mucosa in three (7%) patients. With these high radiation doses (range: 73−95 Gy), they found a 15% increase in local control with every additional 10 Gy over 60 Gy. Stage I to II patients were treated with radiotherapy alone, while Stage III to IV patients received neoadjuvant chemotherapy. For Stage I to IIB patients, 3-year local control and overall survival (OS) were 97% and 67%, respectively, leading the authors to recommend this approach as the standard of care for Stage I to IIB patients, without the need for chemotherapy (20). To address the question of whether intracavitary brachytherapy is valuable in the setting of concurrent chemotherapy, Levendag et al compared their results among T1–2N+ patients treated with neoadjuvant chemotherapy, EBRT to 70 Gy, and a brachytherapy boost to those results obtained with concurrent chemoradiation without a boost (21). They found that for early T-stage patients, local control was significantly improved with the brachytherapy boost: 100% versus 86% without brachytherapy (P = .02). The benefit of intracavitary brachytherapy among early T-stage patients was corroborated by a large study of 509 patients by Teo et al (22). They delivered an intracavitary brachytherapy boost consisting of 18 to 24 Gy in three fractions to 163 patients with locally persistent disease 4 to 6 weeks following completion of EBRT (n = 101) or “adjuvantly” in complete responders (n = 62), resulting in significantly fewer local failures (5% vs 10%) and an improved 5-year disease-specific survival (88% vs 84%) relative to patients who received full-dose RT without a boost. Complications were low with only 10 (6%) patients developing nasopharyngeal ulceration. Another fractionation scheme for HDR intracavitary brachytherapy consisting of two fractions of 5 Gy each, spaced 1 week apart, following (chemo)radiation, was reported for T1 and T2 patients, with a 2-year local control of 94% and no additional toxicity (23). The study by Chang et al is illustrative of the importance of HDR fraction size when HDR brachytherapy boost is utilized for early-stage disease (24). They analyzed 133 patients treated with EBRT to 64.8 to 68.4 Gy, followed by an HDR brachytherapy boost of 5 to 16.5 Gy in one to three fractions, and 50 patients treated with EBRT alone to slightly higher doses of 68.4 to 72 Gy. Although the addition of brachytherapy had a significant local control and survival benefit, 9% experienced palate or sphenoid sinus floor perforation or nasopharynx necrosis, leading the authors to recommend decreasing the fraction size.
Intracavitary or Interstitial Brachytherapy for Recurrent Disease
Several institutional series have reported sustained local control rates of approximately 50% for locally recurrent disease, depending upon the extent of disease and dose administered. Fu et al treated patients with a combination of limited external radiation and brachytherapy, obtaining a 5-year survival rate of 41% (25). Wang reported the results of re-irradiation for 51 patients with recurrent nasopharyngeal carcinoma; T1 and T2 recurrences received an intracavitary boost of 20 Gy after EBRT, and half of these patients were 5-year survivors (26). This study demonstrated the importance of delivering adequate radiation dose (≥ 60 Gy) in the recurrent setting, and reported relatively low morbidity with the integration of brachytherapy. Kwong et al reported the results of 106 patients with persistent or recurrent disease treated with interstitial brachytherapy alone using gold seeds to a dose of 60 Gy, obtaining a 5-year local control rate of 63% and a 5-year OS of 54% for patients with a first recurrence (27). Palatal fistula and mucosal necrosis occurred in 19% and 16% of patients, respectively. Syed et al reported on the use of a brachytherapy implant alone (50–58 Gy) for 34 patients with recurrent or persistent nasopharyngeal carcinoma, with a 5-year local control rate of 59% (28). Late complications were reported in 45% of patients, most often soft tissue necrosis (14%), dysphagia (11%), soft palate atrophy (11%), and nasal crusting (11%). Koutcher et al reported on 29 locally recurrent nasopharyngeal carcinoma patients treated with EBRT with or without the addition of brachytherapy (in 13 patients), consisting of 20 Gy delivered over 2 days using 125I (29). Five-year local control and OS were 52% and 60%, respectively, and did not differ by receipt of brachytherapy. However, the likelihood of late grade 3 or greater toxicity was significantly higher for patients treated with EBRT alone relative to those who received EBRT and brachytherapy (73% vs 8%, respectively); complications for EBRT-alone patients consisted of trismus, temporal lobe necrosis, and cranial neuropathy.
Indications
Intracavitary brachytherapy using a nasopharyngeal applicator may be considered as a boost to address minimal residual disease confined to the nasopharynx following definitive (chemo)radiotherapy. This is appropriate for patients with an early T-stage disease, either T1 tumors or T2 tumors with a sufficient degree of shrinkage following (chemo)radiation, as the depth of the target volume should not exceed 10 mm (10). Tumors invading the base of the skull, infratemporal fossa, oropharynx, or nasal cavities are generally not suitable for this type of brachytherapy. Intracavitary boosts are typically delivered using HDR brachytherapy. According to the Rotterdam experience, patients with T1 tumors were treated with an initial 60 Gy of EBRT, followed by a rest period of 1 to 2 weeks, and then a boost of 17 Gy in five fractions over 3 days. For patients with T2 lesions, 70 Gy of EBRT was followed by a boost dose of 11 Gy delivered in three fractions over 2 days (6 hours apart). Although the Rotterdam group initially boosted T3 to T4 tumors with brachytherapy, the practice is now to deliver a boost to these patients using stereotactic radiotherapy.
Brachytherapy is also indicated for recurrent lesions that are well-circumscribed, superficial, and localized to the nasopharynx. Such lesions can be encompassed using a customized or standardized nasopharyngeal applicator; however, insterstitial brachytherapy has also been used with success. In the setting of recurrent disease in a previously irradiated field, LDR is typically employed, either 60 Gy (over 6 days) if delivered as monotherapy, or 20 Gy (over 2 days) after approximately 45 Gy of EBRT (10,29).
Methods
Various technical approaches are available depending upon the location and extent of disease. Generally, these can be divided into intracavitary and interstitial techniques. Levendag et al have published their method of intracavitary HDR brachytherapy using the silicone Rotterdam nasopharyngeal applicator (Figure 9.3) (20). The applicator is applied after topical anesthesia is applied to the nasal mucosa on an outpatient basis, and remains in situ for the duration of the treatment. It is immobilized to ensure tight apposition between the applicator and nasopharyngeal mucosa. Once in place, dosimetry is based on orthogonal films or CT-based planning. If orthogonal films are used, the dose is generally prescribed to a reference point that is on the midline of the bony surface of the nasopharyngeal roof. Optimization is performed on dose points for both target and normal structures defined on orthogonal radiographs (19). If CT-based dosimetry is utilized, the dose is prescribed to an isodose line covering the surface of the underlying bone. This is generally 5 to 10 mm from the mucosal surface. Radiation is delivered using a remote afterloading 192Ir HDR source. Three-dimensional summation of EBRT and brachytherapy dose is important for accurate dose distribution calculation (Figure 9.4). Other nasopharyngeal applicators utilizing an inflatable balloon or cuffed tip applicator tubes can also be used to help secure the positioning and provide optimal distance from the source to the mucosa for better depth–dose distribution. Intracavitary applicators can also be used to deliver temporary LDR brachytherapy, as described by Koutcher et al in the recurrent setting (29). In an effort to reduce the dose to the soft palate, they used 125I seeds loaded into ribbons inserted into an applicator containing lead shielding.
The use of permanent interstitial implants has been described. For lesions that are discrete and localized, a permanent implant with 125I sources can be highly conformal. However, depending on the location within the nasopharynx, access for interstitial implantation may be difficult, especially for the superior or high posterior wall. Permanent implants have been described using transoral (30), transnasal (31), or transpalatal approaches (32). If the lesion is located in the superior or high posterior wall of the nasopharynx, a transpalatal flap approach can be used (Figures 9.5 and 9.6). A U-shaped incision is created along the hard palate up to the level of the greater palatine neurovascular bundles bilaterally with the creation of a posteriorly based flap, allowing for direct visualization of the superior aspects of the nasopharynx. The greater palatine vessels are preserved with this procedure. A Mick applicator is then used for direct implantation of the tumor. On completion of the implant, a previously custom-made prosthesis would be used to support the reapproximated soft palate for approximately 1 week to allow for adequate healing.

Figure 9.3 The Rotterdam nasopharyngeal applicator. The applicator has been modified to “push” the dose more laterally toward the parapharyngeal space. Courtesy of David N. Teguh, MD, PhD.

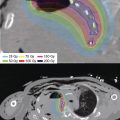
Figure 9.4 Example of dose summation of EBRT and brachytherapy for nasopharyngeal carcinoma. The patient received 46 Gy in 2 Gy fractions to the primary and neck using IMRT, followed by an initial IMRT boost of 24 Gy (2 Gy fractions) to the primary site, followed by an 11 Gy boost using fractionated brachytherapy. EBRT, external beam radiotherapy; IMRT, intensity modulated radiation therapy. Courtesy of David N. Teguh, MD, PhD.
For lesions in the mid and low posterior wall of the nasopharynx, implantation access is through a transoral approach or through the nasal cavity without palatal fenestration. Harrison et al describe a transoral approach with local control of the reported implanted patients (30). After the patient undergoes orotracheal anesthesia, the soft palate is retracted to allow for visualization of the lesion using a dental mirror or a scope and implantation with 125I seeds with a Mick applicator through the oropharynx. A transnasal approach also employs orotracheal anesthesia and soft palate retraction. Using a Mick applicator, permanent seeds would then be implanted under visualization using a telescope in the oropharynx (31).
Figure 9.5 A transpalatal flap approach for nasopharyngeal brachytherapy after the U-shaped incision of the palate with preservation of the greater palatine vessels bilaterally. The sutures on the palate flap help provide the retraction for direct visualization of the nasopharynx. The tongue is retracted with a Dingman mouth gag. Courtesy of Louis B. Harrison, MD.

Figure 9.6 A plain film X-ray of an 125I seed implant of a nasopharyngeal tumor.
Lesions in the lateral or anterior nasopharynx may be more ideally approached using temporary implants because of the anatomic composition and the tendency of the disease for lateral extension into the lateral retropharyngeal space. Harrison et al describe a technique using a specialized applicator with flexible plastic tubes being placed curving toward the side of the nasopharynx (33). After treatment planning has been performed using dummy sources, a hole is drilled at a specific angle allowing for the placement of two angled 192Ir ribbons.
Benefits and Risks
For patients with early T-stage nasopharyngeal carcinoma, and those with locally persistent disease following initial (chemo)radiation, intracavitary HDR brachytherapy delivered as a boost can increase local control. Even in the setting of concurrent chemoradiotherapy, a brachytherapy boost has the potential to augment local control for patients with minimal residual disease confined to the nasopharynx (21). In the setting of recurrent nasopharyngeal carcinoma, the use of salvage LDR brachytherapy, either as monotherapy or as a boost after initial low-dose EBRT, has been shown to result in sustained local control in approximately 50% of patients. Additionally, patients with recurrent disease who are treated with a component of intracavitary brachytherapy as compared to EBRT alone are less likely to experience severe late toxicity.
Potential side effects of intracavitary brachytherapy include the formation of synechiae of the nasal mucosa in less than 10% of patients, and nasopharyngeal ulceration in less than 5% of patients. For patients with recurrent disease, treatment with combined EBRT and intracavitary LDR boost is associated with a low risk of grade 3 toxicity, while interstitial therapy is associated with an approximately 15% risk of soft tissue necrosis, and the risks associated with general anesthesia.
Model Content for Conversation and Consent
Treatment with intracavitary brachytherapy is an outpatient procedure requiring placement of a nasopharyngeal applicator after topical anesthesia of the nares. The applicator is soft and flexible and is generally well tolerated. This will remain in place for approximately 3 to 4 days for the duration of the treatment. Expected side effects include mucositis in the acute setting, and the possible formation of synechiae in the future, which can be minimized by the placement of gauze in the nares.
Interstitial brachytherapy for recurrent disease requires a procedure in the operating room under general anesthesia, which carries its own risks. Potential risks associated with interstitial brachytherapy include hemorrhage and infection; long-term risks include soft tissue necrosis (in approximately 15% of patients), nasal crusting and irritation, bleeding, nasal regurgitation, and dysphagia.
Lip
Cancer of the lip usually presents as an early-stage lesion on the lower lip, and can be effectively treated with either surgery or RT alone. Surgical excision is recommended for small superficial lesions that can be closed primarily without a cosmetic or functional deficit, or for very large tumors that require flap reconstruction. However, excision of intermediate-sized lip cancers, especially with involvement of the commissure, can result in significant cosmetic and functional sequelae. Brachytherapy provides an excellent therapeutic option in comparison to surgery, resulting in equivalent oncologic outcomes with minimal cosmetic and functional disability.
Evidence Basis of the Practice
LDR and Intermediate-Dose-Rate Interstitial Brachytherapy
There are considerable long-term data demonstrating excellent local control (ranging from 90% to 95%) for T1 and T2 lip cancers treated with exclusive interstitial brachytherapy, with good functional preservation and cosmesis (Table 9.1). Jorgensen et al reported a large series of cases of lip cancer, consisting mostly of SCC treated with intermediate dose rate (IDR) brachytherapy using radium needles (34). The authors reported local control rates of 93%, 87%, and 75% for T1, T2, and T3 lesions, respectively, in 766 patients treated with interstitial brachytherapy alone, delivering a total dose of 25 to 56 Gy over 4 hours. The European Group of Brachytherapy reported their results of more than 1,800 cases of lip cancer that were treated with a radioactive implant. The local control was 98.4%, 96.6%, and 89.9% for T1, T2, and T3 lesions, respectively (35). Beauvois et al reported their experience with 237 patients with SCC of the lower lip treated with exclusive LDR brachytherapy using 192Ir, mostly consisting of T1 and T2 lesions (36). Local control at 5 years was 95%, delivering a dose of 65 Gy for superficial tumors (thickness less than 5 mm) and 68 Gy for thicker lesions. Late complications were related to a treated thickness greater than 1.4 cm. Long-term results have been reported by Guibert et al for 92 patients with squamous cell mucosal carcinoma of the lip treated with LDR 192Ir to a dose of 65 Gy (37). Local control was 89%, and nodal recurrences were uncommon (5.4%). The cosmetic and functional results were satisfactory in the majority of patients, 92% and 99%, respectively, with no severe complications such as necrosis. Rio et al reported 95% local control with a lower dose of interstitial LDR brachytherapy, a median dose of 58 Gy, among 89 cases at their institution (38). Objective functional and cosmetic evaluations using a four-item scale including deformity, telangectasias, pigmentation, and skin texture revealed that all patients had retained their initial lip mobility; cosmesis was “good” in 77%, “fair” in 21%, and “poor” in one patient with a T3 tumor. On a multivariate analysis, large tumor size and previous surgery negatively impacted long-term cosmetic results. The local control outcomes reported earlier with exclusive LDR brachytherapy are equivalent to those reported in surgical series, 95% for T1 patients (41).
Table 9.1 Series of squamous cell carcinoma of the lip treated with interstitial brachytherapy alone

HDR Interstitial Brachytherapy
There have been several recent publications analyzing outcomes of HDR brachytherapy for lip cancer (Table 9.1). Ghadjar et al in Bern switched from LDR to HDR in 2004, and reported their results for 33 patients treated with HDR brachytherapy relative to 70 patients receiving LDR implants (39). HDR brachytherapy patients most often received nine fractions of 4 Gy each, delivering 36 Gy in 5 days, while the median dose with LDR was 60 Gy. Oncologic outcomes were equivalent between HDR and LDR patients, with a 5 year local recurrence-free survival of 93% for both groups. With a relatively short median follow-up of 3.1 years, they reported no significant differences with regard to acute or late toxicity between HDR and LDR patients. Overall, 33% of patients experienced late grade 1 toxicity, 5% experienced late grade 2 toxicity, and there was no late grade 3 toxicity observed. Guinot et al compared 104 lip carcinoma patients treated with HDR brachytherapy to 99 LDR patients (40). The majority of HDR patients were treated with nine fractions of 5 Gy each, twice daily, at least 6 hours apart. Ten-year actuarial local control for early tumors (T1 and T2 combined) treated with HDR was 97.5%. With a median follow-up of 46 months among HDR patients, late complications (soft tissue or bone necrosis) were observed in 16% of LDR cases and in no HDR cases (P < .001); good to excellent cosmetic results were found in 89% of LDR patients and 100% of HDR patients (P < .001).
Indications
A simple wedge excision is appropriate for small, superficial tumors measuring less than 5 mm, assuming that primary closure and negative margins can be achieved. Definitive brachytherapy alone is appropriate for early-stage T1 and T2 lip cancers, offering excellent local control with superior functional and cosmetic results than surgery or EBRT alone. Larger tumors (greater than 5 cm) are typically treated with a combination of EBRT and brachytherapy boost. Tumors invading bone are generally treated with surgical resection, if possible. Adjuvant brachytherapy can be employed after surgical resection in the presence of positive margins.
Methods
Interstitial lip implants are typically done under local anesthesia. After the planned implant region has been anesthetized, small catheters are placed in parallel in a single plane with approximately 1 cm spacing (Figure 9.7). The use of flexible plastic catheters as opposed to rigid needles can achieve better conformation to the shape of the lip. Localization films are taken and computer treatment planning performed. For LDR brachytherapy, the catheters are typically loaded with 192Ir or 125I sources delivering the desired radiation dose over several days. During the radiation treatment, the patient wears a dental lead-shielded prosthesis, providing radiation protection to the neighboring mucosa and mandible. In current practice, patients typically receive a total dose of approximately 55 to 60 Gy over 6 days for interstitial brachytherapy alone for early-stage lesions. Patients with T3 tumors can be treated to slightly higher doses. If EBRT is utilized, the lip lesion would receive approximately 50 to 54 Gy followed by an interstitial implant of 20 to 30 Gy over 2 to 3 days.

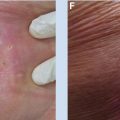
Figure 9.7 The patient presented with a 2.5 cm squamous cell carcinoma of the left lower lip, near the commissure (A). He underwent implantation of afterloading angiocatheters, spaced 1 cm apart, in a single plane. A total dose of 60 Gy was delivered over 6 days, using LDR 192Ir ribbons. This image (B) was taken on day 6 immediately after the removal of the sources, and demonstrates that the lesion has already begun to regress. At 6 months after the implant, the patient has good cosmesis (C) and no evidence of disease.
The implant procedure for HDR brachytherapy is similar to that used for LDR implantation. However, the rigid needle technique with a template can optimize implant geometry, and has been recommended for HDR implants, except in cases with involvement of the commissure or upper lip, which are best treated with the plastic tube technique (10). Treatment planning can be performed using CT images of the implant, treating a clinical target volume (CTV) that includes the tumor with 5 to 10 mm margin. Dose parameters that should be recorded include the V100, V150, D50, and D90, as these have been found to correlate with late toxicity in a dosimetric analysis of patients treated with HDR brachytherapy (39). Typically, treatment would be delivered using an HDR afterloading system with a 192Ir stepping source. Based on the reported literature, nine fractions of 4 to 5 Gy per fraction, delivered twice daily at least 6 hours apart, is a reasonable fractionation scheme; however, the optimal HDR fractionation scheme remains to be determined based on additional long-term data (39,40).
Benefits and Risks
For early-stage lip cancers, interstitial brachytherapy offers a high rate of local control (90%–95%), with good functional and cosmetic outcomes. Good cosmesis has been recorded in 77% to 92% of patients, with the vast majority of patients maintaining their lip mobility. Patients with large tumors or prior surgical resection are more likely to experience less optimal cosmetic results.
Acute skin toxicity including desquamation and inflammation in the irradiated area is expected to occur 2 to 4 weeks after treatment, and to heal within 10 weeks. This is accompanied by mild pain in approximately 70% of patients. Common grade 1 late toxicities include change in pigmentation (15%), fibrosis (15%), and telangectasias (10%–15%). The most common late grade 2 toxicity is pain (5%). With modern techniques, necrosis occurs in less than 5% of patients and resolves spontaneously.
Model Content for Conversation and Consent
• A lip brachytherapy implant procedure can be performed under local anesthesia. Flexible plastic catheters will be inserted into the lip and will remain in place for the duration of the radiation therapy delivery. The risk of significant bleeding and infection with this procedure is low.
• Depending on the dose rate employed (HDR vs LDR), the patient may need to be confined to an isolated room for approximately 6 days.
• In the acute setting, the patient can expect desquamation, inflammation, and mild pain in the area of the implant to occur 2 to 4 weeks after the procedure; these symptoms should subside over the following 2 months.
• Long-term changes to the lip are generally mild and include pigmentation changes and fibrosis. Uncommonly (5%), patients have persistent pain after the implant. Rarely (less than 5%), superficial necrosis may develop, which usually resolves with conservative management over a period of months.
Oral Tongue
SCC of the tongue is the most common malignancy of the oral cavity. Early-stage oral tongue cancer can be treated with either surgery or RT, with comparable rates of local control. Brachytherapy can offer optimal organ and function preservation either as primary therapy, or as adjuvant therapy after a function-sparing surgery. RT is preferably delivered with interstitial brachytherapy either alone, or with EBRT. Brachytherapy alone can be used to treat most T1 or T2 lesions. For larger lesions, a combination of EBRT and brachytherapy is preferred.
Evidence Basis of the Practice
There is a significant body of literature supporting the role of interstitial brachytherapy in the management of oral tongue cancer, both in the definitive and adjuvant setting. The majority of patients have been treated with LDR interstitial implants; however, there are data supporting the use of PDR brachytherapy and an increasing body of literature supporting the use of HDR brachytherapy.
Definitive Treatment With LDR
The largest reported study, with more than 600 patients, of the treatment of T1 to T3 SCC of the oral tongue is by Decroix and Ghossein from the Curie Institute in Paris (Table 9.2) (42). The majority of their patients were treated with interstitial radium implant alone, in particular those with T1, T2, and T3 lesions less than 4.5 cm in diameter, while larger lesions received an implant combined with EBRT or EBRT alone. The radium implants delivered a dose of approximately 70 Gy over 6 to 9 days when given as monotherapy. Larger T3 tumors were treated with a combination of 55 Gy of EBRT followed by an interstitial implant delivering 20 to 30 Gy. The reported local control rates were 86%, 78%, and 71% for T1, T2, and T3 lesions, respectively. The principal complications were soft tissue necrosis in 24.5% and osteoradionecrosis in 14.4% of patients; however, the vast majority of these patients healed with conservative management and only 2% required surgical treatment.
Mazeron et al reported on 166 patients with T1 to 2 lesions treated with interstitial 192Ir brachytherapy alone, using doses ranging from 60 to 70 Gy (43). One hundred fifty-five node negative patients had a 5-year local control rate of 87%. The rates of soft tissue ulceration and bone necrosis in this study were 16% and 12%, respectively, although only 3% required surgical resection. The authors found that both local control and necrosis increased with increasing dose, with marginal improvement in local control with doses greater than 65 Gy; therefore, 65 Gy was their recommended dose when an implant alone is used to treat T1 and T2 tumors of the oral tongue. On a multivariate analysis of the predictors of necrosis, the dose rate and intersource spacing remained predictive of necrosis, and the authors recommended a low dose rate (0.4–0.5 Gy/hr, or 9.6–12 Gy/d) and close intersource spacing (10–14 mm) to minimize this risk (44).

There are several studies demonstrating better local control when a greater proportion of dose is administered with a brachytherapy implant versus EBRT (46,50–52). Wendt et al found that in patients treated with a combination of external and interstitial therapy, the 2-year local control rate was 92% for patients treated with a low dose (< 40 Gy) of EBRT and moderately high-dose brachytherapy (40–55 Gy), versus 65% for patients who received the majority of dose from EBRT (46). They also found that severe complications were more likely in the group of patients who received the majority of their dose from EBRT.
Adjuvant Brachytherapy for Close or Positive Margins
Adjuvant brachytherapy may be considered after excisional biopsy, or for radically resected tumors with close or positive margins, especially if further surgical resection would lead to significant functional disability. Ange et al reported the outcome of 23 patients with oral tongue and FOM malignancies that underwent excisional biopsy (53). These patients were treated with interstitial brachytherapy with doses ranging from 55 to 70 Gy, obtaining 100% local control. However, among the 17 oral tongue patients, 35% developed soft tissue necrosis and 18% developed mandibular necrosis, leading the authors to recommend that the dose not exceed 55 Gy at 12 Gy/d, or 60 Gy delivered at 10 Gy/d. Mendenhall et al reported the results of 16 patients (nine oral tongue and seven FOM cancers) who underwent an excisional biopsy followed by interstitial brachytherapy, either alone or combined with EBRT (54). Local control was achieved in 14 of 15 patients (93.3%); however, 46.7% developed bone exposure as a late complication. Lapeyre et al reported their experience with postoperative brachytherapy alone for 36 patients with T1–T2N0 oral tongue and FOM cancers with close or positive margins following surgery (55). A mean total dose of 60 Gy was delivered at a rate of 15 Gy/d, resulting in 88.5% local control at 2 years. Two patients were subsequently salvaged with surgery and EBRT, yielding an ultimate local control rate of 94.5%. Among the oral tongue patients, 3 of 19 (15.8%) developed transient grade 2 (minor) complications, despite the use of lead shields to protect the mandible. The authors noted that local control was comparable to their experience with definitive brachytherapy alone, with higher rates of grade 2 complications. Biagioli et al reported the results of 22 patients with T1–T2 SCC of the oral tongue treated with interstitial brachytherapy alone after surgical resection with close margins, positive margins, perineural invasion, or lymphovascular invasion (56). These patients received 192Ir LDR to a mean dose of 45.5 Gy. Five-year local control was 95%, with soft tissue ulceration and bone erosion occurring in one patient each.
Chao et al analyzed 55 oral tongue patients treated postoperatively with EBRT, with the addition of an interstitial implant in 16 patients, most often because of involved resection margins (57). They found that local control was not significantly worse for patients with positive margins, and concluded that an interstitial implant converts patients who would otherwise have an ominous outcome due to positive margins to equivalent local control as negative margin patients treated with EBRT.
Experience With PDR Brachytherapy
The largest reported series of interstitial PDR brachytherapy for head and neck cancer was recently updated by Strnad et al to now include 385 patients, of which the majority had cancer of the oral tongue (14). Most patients were treated after minimal surgery, using a dose per pulse of 0.4 to 0.7 Gy (median: 0.55 Gy) delivered 24 hours per day with a time interval of 1 hour between pulses. Patients treated with interstitial PDR brachytherapy alone received a median total dose of 57 Gy. The 5- and 10-year local RFS was comparable to results achieved with LDR—86% and 83%, respectively, with soft tissue necrosis occurring in 10% and bone necrosis in 5% of patients.
Experience With HDR Brachytherapy
With the introduction of HDR techniques, there were concerns that a higher dose per fraction would lead to increased late complications. An HDR schedule derived from the linear quadratic formula of seven fractions of 6.5 Gy delivered twice daily led to relatively low local control (53% at 5 years) among 27 patients with early-stage oral tongue cancer compared to historical controls treated with LDR implants (58). However, Inoue et al simultaneously developed a protocol to treat early mobile-tongue cancer with HDR interstitial brachytherapy using 60 Gy in 10 fractions of 6 Gy delivered twice daily, a regimen that was chosen because it yielded equivalent early mucosal reactions. Based on promising initial results, they conducted a phase III study of HDR versus LDR brachytherapy among 59 patients with T1-T2N0 oral tongue cancer, demonstrating equivalent 5-year local control rates of 87% and 84%, respectively (Table 9.2) (5). There was no significant increase in late effects, with one patient in each group developing a tongue ulcer, and bone exposure occurring in two HDR patients. The authors attributed the low rate of late complications to the small volumes irradiated, and the use of mandibular spacers. In an updated (nonrandomized) analysis of 399 early-stage oral tongue cancer patients treated with either LDR or HDR, the authors reported equivalent 5-year local control rates of 80% and 84%, respectively (59). With a median dose of 70 Gy for LDR patients and 60 Gy (in 10 fractions) for HDR patients, they proposed a conversion factor of 0.86 for HDR in the treatment of early-stage oral tongue cancer. Corroborating the excellent local control achievable with HDR brachytherapy, Leung et al reported a 94.7% local control rate at 4 years for early-stage oral tongue cancer treated with HDR interstitial brachytherapy alone, using a slightly different fractionation scheme of 55 Gy in 10 fractions and a shrinking field technique (47). Only one patient (5%) developed grade 2 soft tissue and bone necrosis. For more advanced lesions, Kakimoto et al reported similar local control for T3 mobile-tongue cancers treated with HDR in comparison to LDR, with a majority receiving combined treatment with EBRT (60). For the 66 patients treated with combination therapy, the median EBRT dose was 30 Gy, followed by a median HDR dose of 48 Gy given in 8 to 10 fractions. Finally, Guinot et al reported their experience with HDR brachytherapy in the adjuvant and perioperative setting, delivering 44 Gy in 4 Gy/fraction when brachytherapy alone was delivered, and 18 Gy in 3 Gy/fraction when delivered after 50 Gy EBRT (61). Local control rates were equivalent to what has been reported with LDR, with soft tissue necrosis in 16% and bone necrosis in 4% of cases.
Brachytherapy for Recurrent or Persistent Disease
Yoshimura et al reported that repeat brachytherapy can be effective for recurrent tumors within the oral cavity (62). They analyzed 62 patients with residual or recurrent oral cavity cancers (71% oral tongue) who had received a prior course of brachytherapy and were managed with repeat brachytherapy consisting of gold-198 (198Au) grains to a median dose of 83 Gy. Local control was 53% at 2 years, with inferior results observed with initial tumors involving a large thickness, or endophytic-type recurrent/residual disease.
Indications
In current practice, interstitial brachytherapy alone can be used to treat early-stage T1N0 and T2N0 cancers of the oral tongue, using a dose of approximately 65 Gy (LDR) at a dose rate of approximately 10 to 12 Gy/d. For larger T2 and T3 lesions, or in the case of node-positive disease, surgery is often the preferred initial approach. If surgery is not undertaken for larger primary lesions, a combination of EBRT and interstitial brachytherapy is preferred. For N0 patients, a dose of approximately 50 Gy in 5 weeks is given to the primary site and neck. After 2 to 3 weeks, an interstitial implant is performed to deliver a boost dose of 20 to 30 Gy. For patients with positive neck disease, 50 Gy is delivered to the primary lesion and upper neck, with a boost of 60 Gy to the gross nodal disease, followed several weeks later by a planned neck dissection and the interstitial tongue implant during the same operative procedure. In the case of close or positive margins after surgical resection, adjuvant interstitial brachytherapy can be delivered as monotherapy to doses ranging from 50 to 60 Gy (LDR), depending on the final margin status. Contraindications to adjuvant brachytherapy include T4 tumors due to bone invasion, or incomplete soft tissue coverage of bone following resection (10).
Methods
There are various technical approaches to an interstitial oral tongue implant. Among the available techniques, the loop technique is often selected (Figures 9.8–9.10). During this operative procedure, the patient would have already undergone any planned neck dissections. Unlike in a base-of-tongue implant, a tracheostomy is not always required for airway protection. If the tumor approaches the base of tongue, some may need a temporary tracheostomy to protect the airway from the tongue swelling and bleeding. Before catheter placement, delineation of the planned number of catheters and entry or exit points as well as identification of various normal structures, including the facial artery, the carotid artery, and the hyoid, are important. A curved metal trocar is introduced into the submental region and aimed toward the intended exit site in the tongue directed by the index finger of the physician’s other hand. Afterloading catheters are threaded through the trocar and then looped over the tongue mucosa and out through the similarly introduced adjacent trocar. The placement of any catheter adjacent to the mandible should be avoided because of the risk of osteoradionecrosis. The spacing between the loops and the adjacent limbs should be approximately 10 to 12 mm, in order to minimize necrosis (44). The exposed catheter limbs can then be tied together within a Penrose drain. Upon completion of the implant, orthogonal verification films are taken with dummy sources in place, and a “loading line” is drawn on the lateral film to delineate the inferior border of the target. Homogeneity of the prescription isodose cloud is optimized by using differential source strengths (1.5–5.0 U [U = unit of air kerma strength = µGy· m2 · h−1]), taking care to avoid overlap of the 166% isodose clouds, using seeds spaced at 1 cm intervals. The dose nonuniformity ratio (DNR, defined as V150/V100) is generally less than 25%. A CT scan can be obtained to delineate the CTV and optimize the source strength and loading pattern to ensure coverage of the CTV and sparing of the adjacent mandible. The catheters are loaded with 192Ir ribbons once patients are comfortable with self-care of a feeding tube and/or tracheostomy care. The dose rate is usually 9 to 10 Gy per day to minimize the risk of necrosis. Patients should wear a custom-designed radiation protective dental prosthesis for added protection to the mandible.
Other common techniques include the guide-gutter technique with iridium hairpins, as described by Mazeron (43), or nonlooping plastic catheters inserted from the submental area through the dorsum of the tongue, affixed in place with buttons on the dorsum of the tongue. The latter technique has been used for HDR interstitial brachytherapy, with the use of a double button to ensure adequate coverage of the dorsum of the tongue (61).
The optimal dose and fractionation for HDR brachytherapy for oral tongue cancer are yet to be defined. In general, treatment should be delivered using a relatively low dose per fraction, approximately 3 to 4 Gy per fraction, twice daily, with at least 6 hours between fractions (10). Given the differences in radiobiological effect and available clinical data, a dose reduction factor of approximately 0.85 is prudent when converting the total prescription dose from LDR to HDR.
Figure 9.8 A patient with squamous cell carcinoma of the oral tongue. Delineation of the target volume and catheter entry sites is performed under anesthesia with a surgical marker.

Figure 9.9 Using the plastic tube looping technique, the target volume is implanted with afterloading catheters.

Figure 9.10 Orthogonal localization films are taken with dummy sources for dosimetric planning. The “loading line” (drawn here in red) indicates the inferior border of the target.
Benefits and Risks
For patients with early-stage T1N0 or T2N0 oral tongue cancer, local control rates with definitive interstitial implant alone are in excess of 90%, and allow for optimal organ preservation. For patients who undergo resection with close or positive margins, adjuvant brachytherapy provides excellent local control and can spare the patient more radical surgery. For patients with larger tumors who cannot undergo surgery, brachytherapy when combined with EBRT is critical to achieve local control.
The two main complications are soft tissue necrosis and osteonecrosis. Soft tissue necrosis occurs in approximately 15% of patients, and is typically a self-limiting process, healing with time; rarely do patients require surgical intervention. Osteoradionecrosis occurs less often, in approximately 5% of patients, but can be severe and may require mandibular resection.
Model Content for Conversation and Consent
• Insertion of the brachytherapy catheters requires an operation under general anesthesia, which carries its own risks.
• A tracheostomy tube may be required for airway protection should the tumor encroach on the posterior tongue. A nasogastric feeding tube will be placed for enteral nutrition during the procedure, and will remain in place until the catheters are removed.
• Acutely, there can be discomfort associated with the catheter placement and tongue swelling.
• There is likely to be mucositis of the tongue 7 to 10 days after interstitial therapy, which takes weeks to resolve. This will likely require analgesics.
• Potential long-term side effects include approximately 15% chance of soft tissue ulceration, which usually heals on its own over a period of weeks to months, but rarely (2%) requires surgery. There is a 5% chance of necrosis of the mandible, which may require surgery. The use of a mandible shield will help prevent this.
Floor of Mouth
As with oral tongue carcinoma, early-stage FOM cancers can be successfully treated with either RT or surgery. Currently, surgical resection is often preferred because high local control rates with excellent functional outcomes can be attained. In addition, the proximity of the mandible to the FOM increases the potential for osteonecrosis if primary radiotherapy is utilized. Brachytherapy can still be appropriately implemented in the treatment of FOM cancer, depending on the clinic scenario, including surgical unfeasibility or medical contraindication.
Evidence Basis of the Practice
When radiotherapy is employed in the management of FOM cancers, there have been numerous reports supporting the use of brachytherapy as part or all of the radiation treatment. Chu and Fletcher compared the outcomes for patients with FOM cancer treated with EBRT alone, brachytherapy alone, or a combination of EBRT and brachytherapy (63). There was significantly improved local control with the use of brachytherapy either alone or in combination with EBRT, resulting in local control rates of 98%, 93%, and 86% for T1, T2, and T3 lesions, respectively. Pernot et al reported their experience with 207 patients with SCC of FOM treated with definitive RT consisting of EBRT and brachytherapy (105 patients) or brachytherapy alone (102 patients) (64). The 5-year local control was 97%, 72%, and 51% for T1, T2, and T3 tumors, respectively. In addition, the authors found that brachytherapy alone yielded superior local control and disease-specific survival for T2N0 patients, with 5-year local control of 92% for brachytherapy alone patients versus 63% for patients receiving a combination of EBRT and brachytherapy (64). There was a reported 6% severe complication rate (requiring surgical resection), with one fatality. Mazeron et al reported their experience with 117 patients with FOM cancer treated with definitive brachytherapy, obtaining primary local control in 93.5% and 74% of T1N0 and T2N0 patients, respectively (45). Tumor size greater than 3 cm and gingival extension were found to negatively affect local control. Matsumoto et al reported similar results for 90 FOM patients undergoing brachytherapy mostly with 198Au grains (65). The local control was 89%, 76%, and 56% for T1, T2a (less than or equal to 3 cm), and T2b (greater than 3 cm), respectively. In addition, the local control was 82% for patients with T1–T2N0 disease without gingival extension, versus 55% for those with gingival involvement. Marsiglia et al reported the Institute Gustave-Roussy experience with 160 patients with T1–T2 FOM cancers less than 3 cm in size who underwent definitive brachytherapy, with a long follow-up of 9 to 19 years (66). The local control rates were 93% and 88% for T1 and T2 lesions, respectively. Any grade of bone necrosis was observed in 18%, with 2.5% requiring hemimandibulectomy. Patients with poor dental status and no dental shield were much more likely to have bone complications.
The risk of osteonecrosis is clearly a major disadvantage of brachytherapy for the treatment of FOM lesions. In general, the risk of osteonecrosis is related to radiation dose, with several studies suggesting EBRT doses greater than 70 to 75 Gy to the mandible predispose to this complication (67,68). Pernot et al reported that for oral cavity and oropharynx cancer patients treated with a component of brachytherapy, FOM location was a significant predictor for bone complications, with an apparent lower dose threshold: for FOM patients treated with brachytherapy alone, there were more bone complications above 68 Gy (7).
Indications
Brachytherapy alone is indicated for T1N0 and T2N0 FOM lesions less than 3 cm in size that are greater than 5 mm from the mandible. Involvement of the mandible is a contraindication to brachytherapy, and gingival extension is generally a contraindication, although tumors with limited gingival extension may still be treated with brachytherapy if surgery is not possible (10). In patients with larger tumors (greater than 3 cm) or tumors in close proximity to the mandible, initial surgical resection is preferred, with consideration of adjuvant radiotherapy as indicated. Because lymph node involvement is common, management of the neck with surgery or radiation is recommended in the majority of cases, except in select T1N0 FOM lesions managed with brachytherapy alone.
Methods
FOM implants are essentially similar to the approach for oral tongue implants. A looping technique is preferred, although the guide-gutter technique can be used for small lesions. The proximity of the FOM to the mandible can pose a significant risk for osteonecrosis, requiring great care during implantation. In addition, proper patient selection, attention to dental care, and use of a lead mandibular shield may help minimize this complication. Caution must be taken regarding the size and placement of the shield to avoid obstruction of the implant and unintended protection of the tumor. A dose of 65 Gy is recommended when brachytherapy alone is employed, while 15 to 25 Gy can be delivered after approximately 45 to 50 Gy EBRT.
Benefits and Risks
Brachytherapy alone for T1N0 and T2N0 FOM lesions yields local control rates of approximately 90%, with excellent aesthetic and functional outcomes. There is an approximately 15% risk of osteonecrosis, with approximately 1% to 3% of patients requiring surgery to address this complication. There is also an approximately 20% risk of soft tissue necrosis.
Model Content for Conversation and Consent
Brachytherapy for FOM lesions usually requires an operation under general anesthesia for placement of the brachytherapy catheters. A neck dissection may be performed at the same time, depending on the size of the primary lesion. After implantation, radioactive sources will be loaded into the catheters and will remain in place for approximately 6 days; during this time the patient will be confined to a private room. A nasogastric feeding tube may be placed for enteral nutrition. In the acute setting, there can be mild-moderate pain associated with catheter placement, and there is a small risk of bleeding and infection. An area of mucositis corresponding to the treatment site usually develops 1 to 2 weeks after brachytherapy treatment is complete, and persists for a period of weeks. In a proportion of patients (~20%), this can develop into soft tissue necrosis, which resolves with conservative (nonsurgical) measures. There is a risk of bone necrosis in approximately 15% of patients, with very few (1%–3%) requiring surgical management.
Buccal Mucosa
Cancer of the buccal mucosa is rare in the Western world. They are more frequently seen in Southeast Asian countries, particularly because of the common use of chewing tobacco- and areca nut-containing products, which are associated with oral cavity cancer. This can lead to oral submucosal fibrosis, which is a precancerous lesion in the mouth and associated with oral cancer (69). Interstitial brachytherapy alone is an option for early-stage lesions. For advanced lesions, surgery or a combination of EBRT and brachytherapy are possible treatment options.
Evidence Basis of the Practice
The largest multicenter study of buccal mucosa cancer was performed by the Groupe Européen de Curiethérapie (GEC), which included 748 patients (70). Treatment of the primary site included brachytherapy alone (31%), brachytherapy with EBRT (11%), EBRT alone (36%), or surgery usually followed by EBRT (22%). Brachytherapy alone was carried out if the lesion was less than 5 cm in size. Local control rates were 81% for brachytherapy alone (n = 266), 65% for combined brachytherapy and EBRT (n = 80), 45% for EBRT alone (n = 273), and 78% for surgery followed by EBRT (n = 167). Another large series was published by Nair et al (71). This series included 234 patients with T1, T2, and T3 buccal mucosa cancer, treated with an interstitial implant to deliver a dose of 65 Gy over a period of 6 days. Stage-specific disease-free survival were 75%, 65%, and 46% for T1, T2, and T3 tumors, respectively.
Indications
Interstitial brachytherapy alone is indicated for small lesions measuring less than 4 cm in size, located in the anterior two thirds of the buccal mucosa, which do not involve the gingiva or the intermaxillary commissure (72). Brachytherapy is contraindicated if there is a deep involvement of the gingivobuccal sulcus, or involvement of the mandible, maxilla, or intermaxillary commissure, due to the high risk of osteonecrosis. Brachytherapy is typically followed by neck dissection for patients at risk for nodal dissemination. For tumors larger than 4 cm in size, or tumors located in the posterior one third of the buccal mucosa (without the contraindications listed earlier), brachytherapy is combined with EBRT. When brachytherapy is contraindicated due to proximity to bone, EBRT or surgical resection with reconstruction is indicated.
Methods
There are several approaches to interstitial implantation of the buccal mucosa. Prior to implantation, the target volume is defined by intraoral examination using bidigital palpation, with one finger in the mouth and the other on the skin of the cheek. The CTV typically consists of the GTV with a 10 mm margin anteriorly and posteriorly, and 5 to 10 mm superiorly and inferiorly depending on the proximity to the adjacent maxilla and mandible (72). MRI is recommended to more accurately define the extent of the gross tumor.
The brachytherapy procedure can be performed under general or local anesthesia. The plastic tube technique is most often utilized for implantation. Angiocatheters or metal trocars are inserted through the skin near the labial commissure in parallel fashion, in an anterior to posterior orientation, transversing through the tumor to exit percutaneously. Under digital control, catheters are positioned 3 to 5 mm deep under the buccal mucosa, with a recommended spacing of 12 to 15 mm (72). The catheters can be secured in place by crimping a metal button at the entrance and exit sites. 192Ir ribbons can then be afterloaded into the catheters. Customized mandibular lead-lined shields should be used along the buccal–alveolar sulcus for providing radiation protection to the surrounding structures including the mandible.
For thin lesions less than or equal to 5 mm, a single-plane implant may be sufficient, while thicker lesions may require a second plane. For lesions involving the posterior buccal mucosa, there is a risk of underestimating the degree of infiltration, and subsequent geographic miss. Furthermore, the isodose curves indent between the wires. To account for this, Lapeyre et al developed a loop technique to encircle the tumor posteriorly (73). A loop is created between the two outside parallel wires, 0.5 cm from the buccal–alveolar sulcus superiorly and inferiorly. They compared their results using either the parallel wire technique or the loop technique for 36 T1–T3 patients treated to a median dose of 65.5Gy (73). The authors reported that 6 out of 14 patients had local failure with the parallel wire technique, compared with 1 out of 22 patients with the loop technique. The 5-year actuarial local control was 91% and 58% for the loop and parallel wire techniques, respectively. There was a 17% incidence of complications observed in 42 patients (in conjunction with patients not in the original comparison) including those occurring after salvage surgery or local recurrence. These complications generally consisted of soft tissue necrosis.
A total dose of 65 to 70 Gy is recommended if LDR brachytherapy alone is prescribed (10). After EBRT delivering a dose of 45 to 50 Gy, a dose of 25 to 30 Gy is delivered as an LDR brachytherapy boost.
Benefits and Risks
Local control rates using 192Ir interstitial LDR brachytherapy alone are approximately 80% to 90% (10). Although there are limited data on the toxicity of brachytherapy for buccal mucosa cancer, complications are estimated to occur in 15% to 20% of patients treated with brachytherapy alone, with less than 10% of patients experiencing grade 3 toxicity, mainly soft tissue necrosis (10,72).
Model Content for Conversation and Consent
The brachytherapy implant is usually performed under general anesthesia. Following implantation, hospital admission in an isolated room is required for 6 days for the duration of the radioactive implant. The risk of soft tissue necrosis is approximately 15%, with a less than 5% risk of osteonecrosis.
Oropharynx
Oropharyngeal cancers pose a unique therapeutic challenge because patients often present at a relatively young age with disease in a critical location that is potentially curable. Management has evolved from an initial surgical approach followed by adjuvant radiotherapy, to one of organ preservation achieved with radiotherapy or concomitant chemoradiation (74,75). With the increasing incidence of oropharyngeal cancers caused by the human papilloma virus (HPV), and an appreciation of the more favorable prognosis of HPV-associated tumors, emphasis has been placed on delivering highly conformal therapy to optimize oncologic and functional outcomes for these patients. At the same time, locoregionally advanced HPV-negative tumors still pose a considerable challenge with regard to obtaining locoregional control. A well-performed brachytherapy implant in the oropharynx provides the opportunity for a highly conformal boost that can optimize oncologic outcomes, while reducing the dose to the pharyngeal constrictors and adjacent critical structures beyond what may be achievable with IMRT (76).
There are considerable data supporting the use of a brachytherapy implant after a reduced dose of definitive EBRT, yielding excellent oncologic and functional outcomes, and there are also data supporting its use after reduced dose chemoradiotherapy. There are accumulating data supporting the use of interstitial brachytherapy as a boost after reduced-dose IMRT. Finally, brachytherapy has been used to treat patients with recurrent or new primary disease within the previously irradiated oropharynx.
Evidence Basis of the Practice
LDR Brachytherapy Boost for Base-of-Tongue Cancers
There are multiple studies reporting excellent oncologic outcomes and acceptable toxicity with reduced-dose EBRT followed by an LDR or PDR interstitial brachytherapy implant for base-of-tongue cancers (Table 9.3). Local control rates from modern series range from 78% for series compromised predominantly of patients with advanced primary tumors, to 94% for patients with earlier stage disease (6,77–79).
Harrison et al reported the long-term results of 68 patients mostly with Stage III or IV base-of-tongue cancer treated with combined EBRT (54 Gy) plus 192Ir implant (20–30 Gy) combined with planned neck dissection for patients presenting with involved nodes (79). Nodal disease was clinically evident in 85% (58/68). Thirteen percent (9/68) who would have required a total laryngectomy, if managed surgically, received induction chemotherapy. No patient received concurrent chemotherapy. At a median follow-up of 36 months, the 5- and 10-year actuarial rates of local control were 89%, distant metastases-free survival was 91%, disease-free survival was 80%, and OS was 86%. All T stages (T1 = 17 patients, T2 = 32 patients, T3 = 17 patients, and T4 = 2 patients) were combined together as there were no differences in local control when analyzed by T stage (T1, 87%; T2, 93%; T3, 82%; and T4, 100% at 5 years). With planned dissection after a reduced dose of EBRT, regional control was 96% at 5 years. Complications after the implant and EBRT occurred in 19% including (a) soft tissue ulcer in 13% (nine patients), all of which healed; (b) osteoradionecrosis in 3% (two patients), requiring mandibulectomy, and (c) brisk bleeding during catheter removal in 4% (three patients), all of which were controlled at the bedside with tamponade and suction. A detailed analysis of patient QOL and performance status found high scores for the three basic functions of eating in public, understandability of speech, and normalcy of diet (80). Additionally, the majority of patients were able to maintain their full-time employment status (81). The primary QOL issue for these patients was xerostomia, which would be expected to be less common nowadays in patients treated with reduced-dose IMRT followed by a brachytherapy implant.
Other series have reported high local control with EBRT and brachytherapy. In an update of the Stanford experience, Gibbs et al reported 5-year local control rates of 86%, 86%, 90%, and 70% for T1 through T4 tumors. The 5-year OS rates were 43% and 71% for Stage I/II and Stage III/IV, respectively (78).
Karakoyun-Celik et al reported the Massachusetts General Hospital (MGH) experience treating 40 base-of-tongue patients, 54% of whom had T3 or T4 tumors, to a slightly higher median EBRT dose of 61.2 Gy, followed by an interstitial 192Ir boost to the primary site using a gold-button single-strand technique, to a total dose of 80 Gy (77). Sixty percent of patients received neoadjuvant chemotherapy; no concurrent chemotherapy was delivered with EBRT. Local control was 78% at 5 years. The majority of late effects were grade 2, with only two patients (5%) developing osteonecrosis.
Of historic interest is the series reported by Housset comparing outcomes using different treatment modalities (82). In this series, T1–T2 patients were treated with EBRT alone, EBRT plus brachytherapy, or surgery plus postoperative RT. The groups were well balanced, except that there were more patients with purely exophytic lesions in the EBRT-alone group. Despite this potentially more favorable unbalance, the local failure rate was twice as high in that group (40% vs 20%). This study suggests that EBRT plus implantation is superior to EBRT alone and is oncologically equivalent to surgery plus postoperative RT. Given the treatment era, these findings may have greater relevance for HPV-negative base-of-tongue tumors.
HDR Brachytherapy Boost for Base of Tongue
Cano et al have reported their experience using HDR brachytherapy after reduced-dose concomitant chemoradiation for base-of-tongue cancers (83). This series consisted of 88 patients, the majority with advanced T3/T4 primary tumors (58%) and clinically palpable cervical nodes (80%), who received concurrent chemoradiation using 3D-CRT techniques to a median dose of 62.4 Gy. Interstitial LDR brachytherapy implants were used for the first 11 patients, and HDR brachytherapy was delivered to the 77 patients that followed. HDR patients received 3 to 3.5 Gy per fraction, twice daily, to a median total dose of 24.5 Gy. The authors implanted cervical nodes as well as the primary site in the base of the tongue, reserving neck dissection for cases of residual neck disease after brachytherapy. The control rates at the primary site and in the neck were excellent at 3 years, 87.5% and 93.2%, respectively. There were low rates of long-term complications, with only four patients developing soft tissue ulceration, and no cases of osteonecrosis.
Interstitial Brachytherapy for Tonsillar and Soft Palate Cancers
Results of interstitial brachytherapy for cancers of the tonsillar fossa and soft palate have been reported using LDR, PDR, and HDR techniques (Table 9.4), with local control rates ranging from 80% to 98%.
Pernot et al developed a plastic tube technique (see the Methods section that follows), and reported the largest series to date consisting of T1–T3 SCCs of the velotonsillar region treated by either a combination of EBRT and brachytherapy (343 patients) or brachytherapy alone (18 patients) with 192Ir LDR brachytherapy (84). The 5- and 10-year local control rates were 80% and 74%, and the OS rates were 53% and 27%, respectively.
Mazeron et al reported the outcome of 165 T1–T2 patients with faucial arch SCC who were treated with either EBRT or brachytherapy, or a combination of both (85). In this study, the 5-year local control was 58%, 100%, and 91% for the three respective groups. Because of the improved local control and survival with brachytherapy, the authors recommended that T1–T2 tumors of the faucial arch be treated with EBRT of 45 Gy followed by a brachytherapy boost of 30 Gy.
Nose et al reported the outcomes of HDR brachytherapy for 83 oropharyngeal carcinoma lesions, the majority originating in the tonsillar fossa or soft palate (86). Most patients received 46 Gy EBRT combined with 21 Gy in 3.5 fractions (6 Gy per fraction) over 2 days, although a minority received HDR brachytherapy alone consisting of 48 Gy in eight fractions. The reported 5-year local control for the patients with tonsillar and soft palate tumors was 86%. Transient soft tissue necrosis occurred in 29%, with six patients (14%) requiring hyperbaric oxygen.
Given the propensity for lymph node dissemination, patients are most often treated with a combination of reduced-dose EBRT and brachytherapy. However, Le Scodan et al treated 44 patients with T1–T2 velotonsillar cancer with interstitial brachytherapy alone (37 patients) or after limited resection (eight patients), including eight patients with a prior history of EBRT for head and neck cancer (87). With a median follow-up of 75 months, the author found only one local failure and four nodal failures, occurring in undissected necks.
What Is the Role of Brachytherapy in the IMRT Era?
The preceding series utilized reduced-dose (chemo)radiation using a 3D-CRT technique, followed by an interstitial implant. In the current era of IMRT-based (chemo)radiation for oropharynx cancer, the role of an interstitial implant has been questioned (88,89). There are emerging data supporting the use of reduced-dose IMRT followed by an interstitial implant to further reduce the dose to surrounding normal structures. An analysis by Teguh et al of 85 oropharynx patients suggests that brachytherapy may provide a means to reduce the dose to the pharyngeal constrictor muscles beyond the sparing that is achievable with IMRT, resulting in less dysphagia (76). This nonrandomized analysis included 85 oropharyngeal cancer patients who received 46 Gy EBRT (using IMRT or 3D-CRT) followed by a boost using either IMRT, interstitial brachytherapy, or stereotactic radiation. For oropharynx patients, boosting with IMRT resulted in more dysphagia than boosting with brachytherapy or stereotactic radiation. The authors found a dose−response relationship between the dose to the superior constrictor muscles and dysphagia, a relationship that has been corroborated by a series analyzing patients treated with either IMRT or brachytherapy (94). The lowest dose of radiation to the swallowing muscles was associated with the use of brachytherapy as a boost. The authors have also found a significant correlation between the radiation dose received by the muscles of mastication (masseter and pterygoid muscles, in particular) and trismus, with patients treated with a brachytherapy boost receiving the lowest dose to these muscles (95). In a recent prospective analysis of patient-reported QOL after (chemo)radiation using IMRT among 207 oropharyngeal cancer patients, the use of brachytherapy as a boost after 46 Gy chemo-IMRT was predictive of better scores on validated scales of dry mouth, swallowing, and opening mouth, relative to the use of IMRT as a boost (90).


Brachytherapy for Recurrent or New Primary Disease in the Previously Irradiated Oropharynx
Mazeron et al reported a 5-year local control of 69% among 70 previously irradiated patients with oropharyngeal cancer treated with 192Ir LDR brachytherapy (97). Patients with tumor involving the soft palate, tonsillar fossa, or posterior pharyngeal wall had 100% local control, while glossotonsillar sulcus and base-of-tongue tumors had worse local control of 69%. Tumor size (>2 cm) also adversely influenced the local control rate, with larger lesions mostly in the base of the tongue. Only 7 of 69 patients (10%) developed nodal relapses. The main complication was soft tissue necrosis (27%) and was self-resolving in 13 of 14 patients. The incidence of this complication appeared to increase among cases in which a large lesion was treated with an implant.
Peiffert et al reported the results of 73 patients with velotonsillar carcinoma in a previously irradiated field treated with 192Ir LDR brachytherapy to a mean dose of 60 Gy (98). The 5-year actuarial local control rates for T1N0 and T2N0 lesions were 80% and 67%, respectively. Grade 2 self-resolving complications, mostly soft tissue necrosis, were observed in 10 patients (13%) who received doses greater than 60 Gy, and there was no grade 3 to 4 toxicity. The 5-year actuarial disease-specific survival and OS were 64% and 30%, respectively, with 42% of patients dying from another carcinoma in the setting of continued alcohol and tobacco use.
The use of interstitial PDR brachytherapy as salvage therapy for unresectable recurrent disease in the head and neck was recently reported by Strnad et al (99). This included 51 patients, the majority with base-of-tongue or mobile-tongue tumors, treated with PDR brachytherapy to a median dose of 60 Gy with concurrent chemotherapy. Two- and 5-year local control rates were 71% and 57%, respectively. Local recurrence-free survival rates were significantly improved in those patients treated with concurrent chemotherapy, 79% versus 39%, respectively at 5 years. Soft tissue necrosis developed in 18% and osteonecrosis in 12% of patients, but only one patient required surgical treatment.
Indications
For base-of-tongue cancers, a brachytherapy implant is typically utilized for patients without significant neck adenopathy, after a reduced dose of (chemo)radiation using IMRT to address the primary site and the bilateral neck. For patients with HPV-negative tumors, which are characterized by decreased radiation sensitivity and inferior local–regional control relative to HPV-positive tumors (101,102), a brachytherapy boost can be an excellent means of increasing dose to the primary site in an effort to improve local control. Regardless of the HPV status, an interstitial brachytherapy implant may be employed to further reduce the radiation dose to the pharyngeal constrictors, muscles of mastication, and other adjacent critical structures (76). Contraindications for a brachytherapy implant include disease extending below the hyoid bone, into the pre-epiglottic space, or invading the epiglottis, as brachytherapy may result in cartilaginous necrosis. Additional contraindications include invasion of the mandible (treatment may result in osteonecrosis) or posterior pharyngeal wall extension.
A brachytherapy implant may also be used for patients with SCC of the tonsillar fossa or soft palate, generally for lesions less than 5 cm in size that are not associated with bulky lymphadenopathy (10). Contraindications to brachytherapy in this setting include tumors involving the retromolar trigone, parapharyngeal space, posterior pharyngeal wall, tumors adjacent to the great vessels or the mandible, or retropharyngeal lymph node involvement.
Brachytherapy is also indicated for patients with recurrent or new primary oropharyngeal cancer in a previously irradiated area. Depending on the extent of disease and its resectability, surgical resection is often attempted, although brachytherapy alone can be used. In those patients who undergo surgical resection, brachytherapy can be used to deliver adjuvant radiotherapy to address pathological risk factors, such as positive margins, while minimizing dose to previously irradiated tissue within the head and neck.
Methods
For base-of-tongue cancers, brachytherapy is typically used as a boost after a course of reduced-dose (chemo)radiation that treats the bilateral neck and the primary site to a dose of 46 to 62 Gy. A dose of 18 to 30 Gy is typically delivered as an LDR 192Ir implant, thereby delivering a cumulative dose to approximately 80 Gy. For T3 and T4 base-of-tongue tumors, higher initial EBRT doses are usually given, followed by a reduced dose implant (10–12 Gy). If HDR brachytherapy is used, a dose of 3 to 4 Gy per fraction is generally recommended with two fractions per day, to a dose of approximately 24 Gy. The use of PDR brachytherapy as described by Levendag et al consists of eight fractions a day, with 3 hour intervals between fractions, delivering a mean total dose of 22 Gy using a mean dose per pulse of 0.56 Gy (6).
For patients with recurrent or new primary base-of-tongue cancer in a previously irradiated area, a dose of 60 Gy using LDR or PDR brachytherapy is often delivered.
Gross nodal disease can be managed in several different ways, including a boost with EBRT to a total dose of 70 Gy, followed by observation in the case of an excellent clinical response, versus neck dissection at the time of the brachytherapy implant in the case of incomplete response. Alternatively, a reduced dose of EBRT is delivered to the neck, followed by planned neck dissection during the same operation as the brachytherapy implant. An alternative strategy described by Cano et al involves implanting both the cervical metastases and the primary site after a reduced dose of chemoradiation, and reserving surgical treatment of the neck for cases of persistent or subsequent lymph node metastases (83).
A base-of-tongue implant is usually done at an interval of 3 to 4 weeks from completion of (chemo)radiation. After induction of general anesthesia and prior to insertion of the brachytherapy catheters, a tracheostomy is placed to protect the airway in case of edema or bleeding. Two techniques that have been described for base-of-tongue brachytherapy include the loop technique, and the gold-button single-strand technique. The loop technique is similar to that used for the oral tongue (Figures 9.8–9.10). When determining the volume of the base of tongue to be covered by the implanted catheters, it is critical that the initial extent of the tumor prior to chemoradiation has been documented. Taking this and any residual disease at the time of the implant into account, a margin of approximately 1 cm is added, taking care not to implant catheters within 5 mm of the epiglottis, lateral pharyngeal wall, or mandible. The loop technique can be used for LDR 192Ir brachytherapy; however, it is generally not used for HDR brachytherapy because the remote afterloading source may have difficulty navigating the loop. The gold-button single-strand technique can be used for either LDR or HDR brachytherapy. Using this technique, a blind-ended afterloading catheter is threaded through the trocar, and seated on the dorsum of the tongue using a gold button, which serves to anchor the catheter and also shield the opposing wall of the oropharynx. If treating with LDR brachytherapy, the distal end of the source strand contains a source to maximize dose to the surface of the base of the tongue (77).
For tonsillar or soft palate tumors, reduced dose IMRT to 45 to 50 Gy is typically delivered to the primary site and draining nodal regions, with concurrent chemotherapy reserved for T3 or N3 tumors, followed several weeks later by an LDR or PDR brachytherapy boost delivering a dose of 25 to 30 Gy (6). The principles discussed earlier for management of the node-positive neck apply.
The most common technique for implantation of the tonsillar pillars or soft palate is the plastic tube technique, described by Pernot et al (84). In the classical Pernot technique, the patient is placed in a seated position with the head in a vertical position. The patient is implanted under conscious sedation with local anesthesia to the skin, tonsillar pillars, and the soft palate. Identification of critical structures, including the hyoid bone, carotid and facial arteries, and the lateral position of the pillars, is performed. Two parallel loops spaced 1.5 to 2 cm apart are implanted to cover the faucial arch. The anterior loop enters 10 mm above the hyoid, and passes through the anterior tonsillar pillar, while the posterior loop enters 10 mm below the hyoid, and passes through the posterior pillar. The posterior catheter is positioned first. A metal guide needle is inserted horizontally below the hyoid bone at a distance from the midline that matches the tonsillar pillar. After transversing approximately 2.5 cm horizontally, the needle is directed superiorly to the base of the posterior pillar. The other free hand is placed in the oropharynx to help direct the needle and ensure that the pharyngeal wall is not punctured. At the base of the left posterior tonsillar pillar, the needle is redirected to the top of the tonsillar pillar along the posterior pillar, finishing with the needle tip piercing the adjacent mucosa. A nylon fishline is then introduced into the mouth through the guide needle and held in place. In a similar manner, another guide needle is percutaneously introduced above the hyoid bone and 1.5 cm from the prior entry point. This guide needle is then tracked up parallel to the other needle along the anterior pillar, ensuring that it is at least 0.5 cm from the mandible. A nylon fishline is similarly placed followed by the removal of the guide needles. Then plastic tubes are introduced with the nylon fishline by clamping the inferior end to the plastic tube at least 10 cm from the end, and then pulled upward intraorally. Fluoroscopy can be done to check placement and parallel alignment. With the same technique, the contralateral tonsillar pillars are implanted with a nylon fish-line. With a specialized long thin curved needle (Reverdin needle), a track is made from the upper exit site of the guide needle along the inferior edge of the soft palate and out through the contralateral side of the uvula. Another nylon fishline is threaded through the needle and left in place. The needle is then reinserted through the same exit site and tracked along the inferior portion of the soft palate and out through the corresponding exit site of the previous guide needle. Another nylon fishline is then left in place. With the laid-out nylon fishline, the plastic tube in the left posterior tonsillar fossa is placed along the soft palate and taken out through the contralateral posterior pillar. These steps are repeated for the anterior half, ensuring parallel alignment with 1.5 cm spacing. If the adjacent base of tongue is involved, additional loops are to be placed under general anesthesia. If the tonsillar fossa lesions are low lying, ipsilateral looping can be performed between the two pillars with the Reverdin needle. On completion, the typical radiation planning would be performed.
Benefits and Risks
A well-performed brachytherapy implant in the oropharynx provides a highly conformal boost that allows for dose escalation to the primary site, while simultaneously reducing dose to the pharyngeal constrictors, muscles of mastication, and other adjacent normal tissues, thereby optimizing long-term functional outcomes. In a patient with recurrent disease or a new primary cancer within the previously irradiated oropharynx, brachytherapy is an ideal way to safely deliver tumoricidal doses of radiation, with reported 5-year local control ranging from 57% to 69%. The risks associated with base-of-tongue brachytherapy include the need for surgery under general anesthesia, and the risks associated with placement of a tracheostomy tube. Long-term risks from the brachytherapy implant include potential soft tissue ulceration, which in modern series occurs in anywhere from 5% to 13% of patients, and osteonecrosis, which occurs in less than 5% of patients. There is a small risk of significant bleeding on removal of the brachytherapy catheters, which occurs less than 5% of the time, and ceases after application of pressure to the brachytherapy catheter sites. For tonsillar pillar or soft palate carcinomas, risks include soft tissue ulceration in 5% to 20% of patients, which is generally transient; the risk of grade 3 soft tissue necrosis may be higher if HDR brachytherapy with a high dose per fraction is utilized (86).
Model Content for Conversation and Consent
• Insertion of the brachytherapy catheters requires an operation under general anesthesia, which carries its own risks.
• A tracheostomy tube is required for airway protection, and will be placed during surgery prior to placement of the brachytherapy catheters. This will usually be removed the day of, or the day after, removal of the brachytherapy catheters.
• If a feeding tube has not already been placed, a nasogastric feeding tube will be placed for enteral nutrition during the procedure, and will remain in place until the catheters are removed.
• Acutely, there can be discomfort associated with the catheter placement.
• Depending on the dose to be administered with a brachytherapy boost, and the dose rate employed, the patient will need to be admitted for approximately 5 days. If LDR brachytherapy is employed, the patient will need to be confined to a private room during the period of time that the radioactive sources are loaded. This is not a concern if HDR or PDR brachytherapy is employed.
• There is likely to be mucositis of the treated oropharyngeal mucosa occurring approximately 7 to 10 days after interstitial therapy, which takes weeks to resolve. This may require analgesics and dietary modification.
• Potential long-term side effects include an approximately 5% to 15% chance of soft tissue ulceration, which heals on its own over a period of weeks to months. Approximately 5% of patients may require hyperbaric oxygen to accelerate healing of the ulcer.
• There is less than a 5% chance of necrosis of the mandible, which may require surgery.
Recurrent SCC in the Head and Neck
Recurrent cancer in a previously irradiated field poses a difficult challenge as patients have often received extensive multidisciplinary treatment, and present with biologically aggressive locoregional disease, and are at high risk for distant metastases. In addition, retreatment is associated with high rates of severe complications. Balancing these risks with the limited chance for salvage requires careful patient selection and meticulous multidisciplinary evaluation. Before initiating any therapy, patients should undergo a complete staging workup to help determine the overall treatment strategy and the “curability” of the disease, in addition to assessing the patient’s overall medical condition and performance status.
Treatment options vary depending on the site of recurrence. Typically, the overall strategy requires evaluation for resectability and ruling out distant metastases. If resectable, surgery is generally recommended even if sacrifice of adjacent structures, such as the larynx, is required for oncologic clearance. However, salvage surgery alone is often inadequate, and adjuvant radiation may be indicated as patients remain at high risk of recurrence. For unresectable tumors, re-irradiation with EBRT and/or chemotherapy is possible, but with increased risk of severe toxicities such as pain, wound breakdown, carotid artery hemorrhage, infection, fistula formation, and necrosis (103–107). Given its high degree of conformality, brachytherapy provides an attractive treatment option either as adjuvant therapy or as definitive therapy for recurrent or de novo tumors arising in previously irradiated patients.
Intraoperative radiation therapy (IORT) is another attractive approach to deliver HDR brachytherapy. This technique refers to the application of a single high dose of radiation during an operative procedure, usually after maximal resection of gross tumor. Direct visualization of the tumor bed at risk is possible, while neighboring normal tissues can be displaced or shielded, thereby optimizing dose delivery. An additional benefit is the opportunity to treat when the tumor burden is at its lowest. Reconstruction with a nonirradiated soft tissue flap at the time of IORT or brachytherapy implant can reduce the risk of wound complications (108,109).
Evidence Basis of the Practice
The evidence supporting the use of brachytherapy for recurrent disease within the nasopharynx, oral cavity, and oropharynx is reviewed within these respective sections (see earlier discussion). Here we summarize the experience using brachytherapy or IORT to address recurrent disease in the previously irradiated neck.
Interstitial Brachytherapy Following Resection for Recurrent Neck Disease
Goffinet et al described a permanent neck implant using 125I seeds impregnated in a vicryl suture material, as adjuvant therapy after resection (110). For 34 previously irradiated patients with recurrent disease, local control was obtained in the implanted volume in 59%. Complications developed in 20% of patients, leading the authors to recommend limiting the total interstitial dose to 120 to 140 Gy.
Choo et al reported on 20 patients with recurrent or persistent neck metastases treated with 192Ir implants (111). In addition to an implant, treatment consisted of salvage surgery for nine patients, combined EBRT for three patients, and brachytherapy alone for eight patients with large unresectable neck nodes measuring 5 to 10 cm. They reported that 15 patients had complete clearance of tumor, and 13 were controlled at the time of death or last follow-up.
Cornes et al at the Royal Marsden Hospital analyzed the outcome for 39 patients with inoperable neck nodes, who underwent maximal surgical resection and re-irradiation with implantation of 192Ir seeds with or without a reconstruction flap (112). In the 13 patients without reconstruction, the local control rate at 1 year was 68%. However, 46% also experienced severe radiation-induced fibrosis and neck contracture. In the remaining 26 patients who underwent flap reconstruction, local control at 1 year was 63%, with significant morbidity in 12%. These investigators updated their experience using 192Ir LDR brachytherapy after subtotal excision and flap reconstruction for 74 patients with recurrent neck disease (113). Brachytherapy was delivered to the tumor bed to a dose of 60 Gy. Reported in-field local control rates ranged from 40% to 62% at 2 years and 40% to 58% at 5 years. The best results were obtained with surgical excision, brachytherapy, and myocutaneous flap reconstruction, with 72% and 66% in-field local control at 2 and 5 years, respectively. The overall major complication rate was 15%, including fistula, hemorrhage, and wound breakdown.
Kupferman et al reported the MD Anderson experience treating 22 patients with recurrent neck disease with neck dissection followed by intraoperative placement of afterloading catheters, used to deliver 60 Gy over 4 days (114). The majority (19/22) also had a muscle flap reconstruction for soft tissue coverage. The 2-year actuarial regional control rate was 67%, with few postoperative complications.
Interstitial HDR brachytherapy has been used after neck dissection, as reported by Pellizzon and colleagues, for 42 patients, 35 of whom received previous EBRT (115). The median HDR dose was 24 Gy in six fractions. Five-year relapse-free survival (RFS) and overall survival (OS) were 49% and 53%, respectively.
Narayana et al reported results of HDR interstitial brachytherapy among 30 patients with recurrent head and neck cancer (116). For the 18 patients who underwent surgical resection followed by brachytherapy, 3.4 Gy was delivered twice daily to a total dose of 34 Gy, while nine patients treated with brachytherapy alone received 4 Gy per fraction BID to 40 Gy. Two-year local control and OS were 71% and 63%, respectively. Corroborating other studies showing improved local control with resection, 2-year local control was 88% among those treated with surgical resection and brachytherapy, versus 40% for those treated with brachytherapy ± EBRT alone (P = 0.05).
Interstitial Brachytherapy Alone for Recurrent Neck Disease
Brachytherapy alone has been utilized for select patients with unresectable recurrent neck disease, with mixed results.
Bollet reported on 84 patients with isolated cervical lymph node relapses treated mostly with brachytherapy alone (72 patients to a mean dose of 56.5 Gy) or a combination of EBRT and brachytherapy (12 patients with mean brachytherapy dose of 38 and 41 Gy EBRT) without surgery (117). Long-term lymph node control rates were disappointing, 31.5% at 2 years and 0% at 5 years. If the total dose was greater than or equal to 60 Gy, the control improved to 56% at 3 years. Significant toxicity occurred in 35%, with 7% being fatal. The authors concluded that salvage surgery should always be performed when possible.
Puthawala published a large series of 220 patients with recurrent head and neck cancer treated with interstitial LDR brachytherapy, the majority (54%) with recurrent metastatic neck disease (100). Patients received a median minimum tumor dose of 53 Gy with brachytherapy; 60% also received interstitial hyperthermia, and 40% concurrent chemotherapy. The authors reported cumulative local control of 77%, 69%, and 51% at 6 months, 2 years, and 5 years, respectively. The disease-free survival rates at 2- and 5-years were 60% and 33%, respectively. Moderate to severe complications were seen in 27%, most often soft tissue necrosis and osteonecrosis.
Hepel et al reported the outcomes of 30 patients with recurrent, previously irradiated head and neck cancer, the neck being the most frequently implanted site (118). All patients were inoperable, refused surgery, or had gross residual disease after attempted salvage surgery. Patients were treated with HDR interstitial brachytherapy to a mean dose of 34 Gy in twice-a-day fractions of 3 to 4 Gy. With a minimum follow-up of 12 months, the local control rate was 69%, with 2-year OS of 37%. The rate of grade 3 to 4 complications was 16%.
Tselis et al reported on 74 patients with inoperable neck recurrences treated with CT-guided interstitial 192Ir HDR brachytherapy (119). Catheter implantation was performed under interactive CT guidance, which was followed by contrast-enhanced CT for 3D treatment planning. Almost all patients received twice-daily fractions of 3 Gy per fraction to a median dose of 30 Gy. OS at 1 and 2 years was 42% and 19%, respectively, with corresponding local control of 67% at both time points. Late grade 3 or 4 toxicity was reported in 8%, most often fistulae.
Intraoperative Radiation Therapy
The early experience of IORT for recurrent or locally advanced head and neck tumors typically employed intraoperative electron therapy (IOERT) delivered after maximal surgical resection, to doses of 15 to 30 Gy without additional EBRT (120–124). Taken together, these studies demonstrated that IORT conferred reasonable local control rates for patients with close margins or microscopic residual disease after resection, but could not be expected to control gross disease. For example, Toita et al found that although the 2-year in-field local control rate was 54% overall, it varied significantly according to the extent of resection: 0% in patients with gross residual disease, 55% in cases of microscopic residual disease, and 82% in patients with close margins (122). Another common finding reported in several studies was an increased risk of complications, in particular carotid artery hemorrhage and neuropathy, with IORT doses more than 20 Gy (122,124).
More recent studies employing IOERT include an analysis by Chen et al of 137 patients with recurrent or persistent head and neck cancer, 83% having received prior radiation (125). Patients received a single fraction of electrons to 15 Gy after gross total resection. The 1-year, 2-year, and 3-year rates of in-field control were 70%, 64%, and 61%, respectively. As in other series, positive margins at the time of IORT predicted for subsequent in-field failure. There were low rates of complications: 3% wound infection, orocutaneous fistula in two patients, and flap necrosis, trismus, and neuropathy in one patient each.
Zeidan et al reported on 231 patients who underwent neck dissection and IOERT for advanced cervical metastases, the majority (88%) in the salvage setting (126). Patients received 15 or 20 Gy using 5 MeV electrons. The reported 5-year RFS and OS were 49% and 26%, respectively. Complications occurred in 27% of patients including vascular complications in 11%, fistulae in 10%, and wound dehiscence in 10%. Patients treated with doses above 15 Gy had significantly improved RFS. The same authors also reported their experience using IOERT as adjuvant therapy after gross total resection for primary or recurrent parotid gland cancer, with only one patient experiencing local recurrence (127).
Several institutions have reported encouraging results using the Harrison–Anderson–Mick (HAM) applicator connected to a remote afterloader with an HDR 192Ir source (HDR-IORT) as a means to deliver intraoperative brachytherapy directly to the at-risk tumor bed.
Scala et al reported the Beth Israel Medical Center experience of 76 patients treated on 87 sites with HDR-IORT (128). All patients underwent gross total resection, followed by a median IORT dose of 12 Gy (range: 7.5–17.5 Gy). Postoperatively, 24% of patients received additional EBRT to a median dose of 45 Gy, indications being positive pathological margins, multiple involved nodes, or extranodal disease extension. The 2-year in-field control rate estimate was 62%, with in-field control varying by the total biological effective dose (BED) received: 84% versus 54% for greater than versus less than the median total BED, respectively. Additionally, significantly longer survival was reported for patients achieving in-field control. These findings have altered the group’s current practice, which is now to deliver 15 Gy intraoperatively if possible, routinely supplemented with EBRT for patients at high risk of relapse. Myocutanous flap reconstruction is often performed at the time of resection and IORT to introduce well-vascularized tissue, based on data showing the feasibility of this approach (129). Using this approach, there were low rates of complications including 4% risk of flap revision, 1% nonfatal carotid hemorrhage, and 1% vagal neuropathy (128).
Indications
For recurrent tumors within the head and neck, surgical resection should be performed if possible, as this appears to confer the highest probability of locoregional control. However, even after maximal safe resection, these patients remain at risk for recurrence, and adjuvant radiotherapy is often indicated based on the likelihood of microscopic residual disease. Depending on institutional experience, adjuvant radiation can be safely delivered to the resection bed with interstitial brachytherapy or at the time of surgery using IORT. IORT is indicated only if gross total resection can be performed, given the evidence that IORT alone is unlikely to control gross disease. At the authors’ institution, after gross total resection of recurrent disease, HDR-IORT is delivered to the operative bed, followed by myocutaneous flap reconstruction. Additional EBRT is often indicated based on the final pathological risk factors, including involvement of multiple lymph nodes, invasion of adjacent structures, or margin uncertainty.
Concurrent radiosensitizing chemotherapy is often recommended for positive margins or nodal extracapsular extension.
In patients with unresectable recurrent disease, interstitial brachytherapy offers the potential for sustained local control with acceptable toxicity.
Methods
The technical approach varies depending on the extent of disease, location of tumor, presence of critical normal structures, surgical accessibility, and prior radiotherapy.
Interstitial implants, either temporary or permanent, can be performed using a variety of approaches. Permanent implants using low-activity 125I seeds have historically been preferred when there is residual gross disease or involvement of a vital structure such as the carotid artery in the neck, which may not be resectable. With the use of a Mick applicator, direct volume implants can be performed into residual gross tumor. The Anderson nomogram can estimate the number of seeds on the basis of seed activity and the average dimension of the volume implant (132). Delineation of the volume of disease is crucial to ensure a successful implant. The seeds are usually spaced approximately 1 cm apart. On completion, dosimetric calculations are performed using a CT scan. Suture seeds can also be used for a single plane implant. The suture seeds are sewn directly into the tumor bed, typically 1 cm apart. This may be ideal in cases following gross total resection where there is a high risk of residual microscopic disease. If the disease is resected adjacent to the carotid artery, a permanent implant can be facilitated by the use of a Dexon mesh. The suture seeds are sewn directly into the mesh in a parallel fashion. The mesh is then implanted over the entire tumor bed. Typically, a dose of 140 Gy is delivered to the tumor bed, if possible. With either method, a nonirradiated soft tissue flap should be used for reconstruction of the neck to minimize any potential re-irradiation complication such as a carotid blowout.
In addition to permanent implants, temporary implants can be performed to facilitate delivery of HDR or LDR brachytherapy. Various techniques have been described. For unresectable gross disease within the neck, Tselis et al have described a method of interactive CT-guided catheter implantation, followed by a contrast-enhanced CT for volume-based planning (119). The published HDR experience for inoperable gross recurrent disease within the neck would support delivering twice-daily fractions of 3 to 4 Gy each, to total doses of 30 to 34 Gy. In cases of proximity to the oropharyngeal mucosa, Tselis et al used fractional doses less than or equal to 3 Gy.
For patients undergoing neck dissection, the placement of afterloading catheters is performed in the operating room after maximal gross total resection. The surgical tumor bed is reviewed with the surgeon, and clips are used to demarcate the planned treatment region. Using an approach similar to the plastic tube technique, afterloading catheters are placed over the tumor bed. The orientation of the implant is such that the catheters are spaced far enough from the suture line to minimize wound-healing complications, and so that there is minimal physical displacement after reconstruction and during patient movement. The planned skin entry sites are demarcated to be approximately 1 cm apart to cover the tumor bed with adequate margin. The proximal ends of the catheters should be placed beyond the surgical bed to ensure proper coverage with margin. If flap closure is planned, the catheter entry sites are placed as far laterally as possible in order to accommodate the flap, which is placed over the catheters prior to skin closure. If treating with LDR, the catheters are secured with metal buttons, crimped and sewn to the adjacent skin, and the exposed ends of the catheters are cut with ample remaining length and secured with a Penrose drain. If treating with HDR, the blind ends of the catheters are seated anteriorly using half-moon buttons, rubber buttons are brought up to the skin over the other end of the catheter, and the free posterior ends of the catheters are secured until simulation.
Radiation planning can be performed using orthogonal films, or CT-based volumetric planning. The patient is loaded after 5 or more days to allow for adequate healing. When using LDR brachytherapy, a dose of 60 Gy is recommended, as for other recurrent head and neck sites. Although there are limited data regarding the optimal dose and fractionation for HDR brachytherapy in this setting, one fractionation scheme is 3.4 Gy per fraction to a total dose of 34 Gy using HDR brachytherapy alone (116). Alternatively, an HDR boost can be integrated with additional EBRT, typically in the range of 45 Gy.
Intraoperative Radiation Therapy
Traditionally, IORT was delivered using electrons. However, the use of a linear accelerator in a dedicated shielded operating room is costly, and the alternative of intraoperative transportation to the radiation oncology department is logistically challenging.
The intraoperative delivery technique favored by the authors is the utilization of an HDR remote afterloader with 192Ir sources, which can provide an advantage with regard to expense, mobility, and the absence of unwieldy applicators. A shielded room with radiation security monitoring is required for treatment, in addition to an adjacent “clean” room for observation of the patient during treatment. Several intraoperative applicators are available, which conform to curved surfaces and can be resized to match the operative bed. For example, the HAM applicator is a specialized 0.8 cm-thick pad of transparent flexible material with embedded source guides, which can be attached to the remote HDR afterloading system. The number of channels can be customized depending on the area to be treated. The distance between the embedded catheter and the treatment surface is 0.5 cm, allowing for acceptable dose uniformity at the surgical bed surface while maximizing the dose falloff with depth.
Multidisciplinary care is essential for successful IORT treatment. After gross total resection, the operative bed is reviewed with the surgeon who can assist in the identification of critical structures such as cranial nerves, major blood vessels, and potential sites of microscopic disease. In addition, the vascular pedicle of the harvested myocutaneous flap that will be used for closure is identified and displaced from the operative bed. An appropriate-sized applicator is placed on the tumor bed and secured into place with suturing and/or gauze packing. An accurate measurement of the tumor bed is taken to determine the number of catheters, source dwell positions, and the overall treatment plan. The dose to critical structures, such as nerves, bone, and major blood vessels, is reduced by physical displacement, or through careful placement and immobilization of lead shields. Care is taken not to place the applicator directly on hollow viscera such as the esophagus or pharyngeal wall. The typical dose delivered through IORT is usually 12 to 15 Gy. IORT is followed by myocutaneous flap reconstruction in the majority of cases, to introduce a fresh vascular supply to the tumor bed. Based on the final pathological findings, additional EBRT to 45 Gy would be delivered approximately 4 weeks postoperatively.
Benefits and Risks
For patients with recurrent head and neck cancer in a previously irradiated field, with no evidence of metastatic disease, consideration is given to aggressive salvage therapy because significantly longer survival has been reported for those patients achieving in-field control. The best results are obtained when surgical resection is combined with adjuvant radiation, with reported 2-year in-field control rates ranging from 62% to 88% in series utilizing adjuvant interstitial brachytherapy or IORT. Major complications from re-irradiation in this setting include fistula, carotid artery hemorrhage, wound breakdown, and osteonecrosis within the treatment field. In patients treated with interstitial brachytherapy, major complication rates of 15% have been reported, most often wound breakdown, fistula, or osteonecrosis. In patients treated with IORT, complication rates are generally quite low if the dose is kept below 20 Gy. In series utilizing IORT followed by supplemental consolidative EBRT in a proportion of patients, the risk of complications is low in patients who undergo myocutaneous flap reconstruction; in particular, a less than 5% risk of wound breakdown requiring flap revision, hemorrhage, or cranial neuropathy (128).
For patients with unresectable recurrent neck disease, interstitial brachytherapy can be used to obtain local control, with 2-year in-field control rates ranging from 32% to 69% in several series. However, OS for these patients remains poor, with 2-year estimates ranging from 19% to 37%. Complication rates are higher in this setting, with rates of severe toxicity reported in 16% to 27% of patients, most often soft tissue necrosis, osteonecrosis, or fistula formation.
Model Content for Conversation and Consent
The discussion and consent process will vary considerably depending on whether the patient is going to be treated with interstitial brachytherapy or IORT.
For patients who are going to be treated with IORT following gross total resection of recurrent disease, the following points are made:
• Radiation is delivered to the tumor bed during one operative procedure, following resection, and prior to flap reconstruction. A separate operative procedure is not required. The length of hospital stay is generally not affected by delivery of IORT.
• Depending on the location and extent of the recurrent disease, there are few acute risks associated with IORT; however, there is the potential for severe and life-threatening subacute or late complications including carotid artery hemorrhage, cranial nerve injury, fistula formation, bone necrosis, or wound breakdown requiring flap revision. Generally, the risk of these complications is less than 5%, and will be minimized during the procedure by physical displacement of critical structures, and the use of lead shields.
• Approximately 1 month following surgery, consideration will be given to supplemental adjuvant EBRT to the tumor bed, depending on final pathological risk factors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree