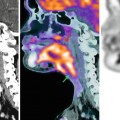
Fig. 40.1
Typical vascular pattern for hepatocellular carcinoma (HCC) (arrows) seen at contrast-enhanced ultrasound (CEUS). The nodular lesion appears as hypoechoic lesion at grey-scale ultrasound (a). After intravenous contrast injection, the nodule depicts arterial enhancement (b) followed by contrast washout on delayed venous phase (c). Although this pattern is typical for HCC, it is not specific for HCC
Acoustic radiation force impulse imaging (ARFI) is a newly developed technique for the evaluation of tissue stiffness. It has been applied to evaluate liver fibrosis, but there is only limited data available for liver lesions characterization. In those preliminary data, ARFI measurements alone barely can differentiate benign and malignant liver nodules because of variations in stiffness of all types of masses, but cannot differentiate HCC from other malignancies [20, 21]. The substantial overlap between values of benign and malignant liver masses limits clinical usefulness of this technique.
On unenhanced CT scans, HCC often appears as a hypodense lesion. Typical HCC shows marked transient hyperenhancement compared to liver background during late arterial phase on dynamic studies. Typical HCC rapidly becomes iso- or hypoattenuating during portal or delayed venous phase [19, 22, 23]. Additional features on CT that may be associated to nodular HCC are the detection of a thin hyperenhancing tumoral capsule in portal or delayed venous phase [22, 24]; the presence of mosaic pattern, especially in large tumors, with different internal areas of differentiation[22, 24]; and the expansive vein thrombosis.
HCC has variable signal intensity on MRI, but predominantly it shows low signal intensity (SI) on T1-weighted imaging and high SI on T2-weighted imaging. Small HCCs may present fatty metamorphosis, causing hyperintense SI on T1-weighted images. Fat content can be confirmed when the SI of the lesion drops on opposed-phase imaging relative to in-phase imaging [25]. HCC can be difficult to detect on T2-weighted images because of heterogeneity of the cirrhotic liver, which obscures mildly hyperintense and isointense tumors.
On dynamic phases of gadolinium-based MRI, typical HCCs use to show hyperenhancement in the hepatic arterial phase compared to the surrounding liver parenchyma followed by hypointensity (washout) in the venous and/or delayed phases (Fig. 40.2). The fast decrease of SI on the venous and/or on delayed phases has been attributed to the loss of portal-venous supply at the latest stages of hepatocarcinogenesis [26, 27]. In a low proportion HCCs may be hypoenhancing and nearly isointense in arterial phase and persists with low SI during venous phases.
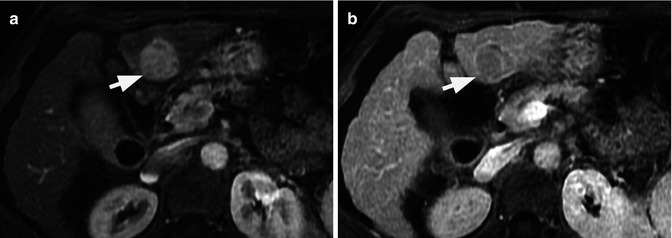

Fig. 40.2
Typical MRI vascular pattern for hepatocellular carcinoma (arrows). The nodular lesion depicts typical hyperenhancement of gadolinium on arterial phase (a) followed by contrast washout on delayed phase (b)
Rarely, HCCs may remain hyperintense relative to liver parenchyma on the delayed phases. This atypical vascular pattern had been described in scirrhous HCCs and use to be secondary to the presence of prominent central fibrous stroma [28].
MRI has been reported to be more sensitive than CT to depict the peritumoral fibrous capsule or pseudocapsule, which is a frequent finding on HCC. Both capsule and pseudocapsule appear on MRI as thin rim that surrounds the HCC, presenting hypersignal on T2 and hyperenhancement on delayed venous phase [29]. This fact should represent a helpful diagnostic sign in cases of atypical vascular pattern. However, the presence of pseudocapsule can be identified in a small proportion of atypical nodules that finally resulted HCCs [30].
Recent studies have shown that the detection of all types of focal liver lesions, including small HCCs, is improved with diffusion-weighted imaging (DWI) compared with conventional T2-weighted imaging [31, 32]. In highly cellular tissues such as HCC or other malignant lesions, diffusion of water protons is impeded within the lesion, resulting in high SI of the lesion versus background liver [33]. DWI is now a routine part of all liver MRI protocols and may improve the detection of HCC or reader’s confidence in the diagnosis of HCC. Similar to T2-weighted, hyperintense SI on DWI is not specific for HCC, even in the cirrhotic liver, and other focal liver lesions may show this appearance, such as intrahepatic cholangiocarcinoma (ICC) [34].
Recently, An et al. [35] reported that DWI together with dynamic MRI phases might be useful to predict the histological grade of HCC. The authors found a tendency towards the higher grades of HCC showing the more restriction on DWI. Also, the results from this study showed that characteristics from DWI and subtraction of unenhanced images from arterial phase images can be used to predict the HCC histological grade, especially when SI characteristics on both MRI sequences are considered together: poorly differentiated HCC showed higher SI on DWI and/or arterial enhancement on subtraction imaging [35] (Fig. 40.3). In spite of these are novel results, the impact of histological grade of HCC over the decision-making process is still under discussion.
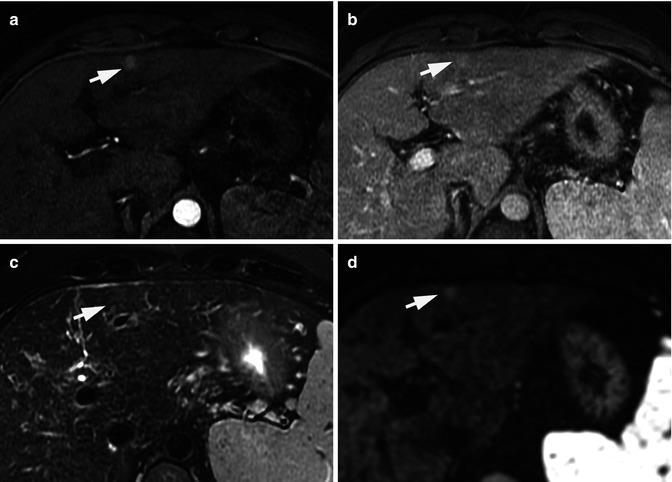

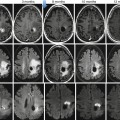
Fig. 40.3
Atypical vascular pattern for a hepatocellular carcinoma. Fifteen mm hepatic nodule (arrows) that enhances on the arterial phase (a) but does not display venous washout (b). The nodule depicts iso-signal intensity on T2-weighted image (c) and high signal intensity on DWI (b = 800 s/mm2) (d). The lesion underwent a biopsy. Histological section (hematoxylin-eosin stain) from cellblock obtained with fine-needle aspiration cytology was diagnostic of a well-differentiated hepatocellular carcinoma
An important technical aspect to be considered for interpreting the findings on DWI is that the sensitivity for HCC detection on DWI decreases with increasing severity of cirrhosis since the SI of the liver background increases and the liver to HCC contrast decreases [36]. As a general rule, the apparent diffusion coefficient (ADC) quantification of malignant liver lesions, including HCC, is lower than benign lesions [37], but the overlap on ADC values found among different liver malignancies and premalignant conditions, such as dysplastic nodules, and the heterogeneity among different studies regarding the technical characteristics on DWI sequences represent a major limitation at the time to establish a threshold to define the ADC value for HCC characterization [33, 37].
One of the major changes in liver imaging in the past years is the introduction of gadolinium-based contrast agents with liver-specific properties including gadobenate dimeglumine and gadoxetic acid. Due to the higher biliary excretion of gadoxetic acid (50 %) compared to gadobenate dimeglumine (3–5 %), gadoxetic acid is more often reported in the literature. Both contrast media provide information regarding tumor vascularity during the arterial and portal-venous phases of the study and functional information about the presence or absence of functional hepatocytes during the hepatobiliary (HB) phases generally obtained 20 min after gadoxetic acid injection.
The ultimate factor that conditions the gadoxetic acid uptake during the HB phase is the expression of two different transport systems located at the sinusoidal and canalicular membranes of the cell, including the OATP1B1 and OATP1B3, a solute carrier transporter, and the multidrug resistance protein 2 (MRP2), a canalicular transporter [38]. Therefore, non-hepatocyte focal lesions or those with nonfunctional hepatocytes, such as most HCCs, will not accumulate contrast, resulting in low-signal-intensity foci relative to the high SI enhancing liver parenchyma in the HB phase (Fig. 40.4). The percentage of the gadoxetic acid that is not cleared by the hepatobiliary system is excreted by glomerular filtration in the kidneys. Therefore, the liver uptake on HB is surrogated by the renal and liver function [39]. Even though several authors have attempted to find biological markers to predict a qualitative enhancement on the HB phase, including indocyanine green, prothrombin, albumin, liver enzymes, and Child-Pugh score, there is no clear biological marker that is consistently associated with reduced uptake of liver parenchyma on the HB phase. Nevertheless, the general trend shows reduced liver enhancement on the HB phase with increasing severity of cirrhosis, but not all patients with severe cirrhosis (Child B and C) had reduced uptake of contrast material [39–42].
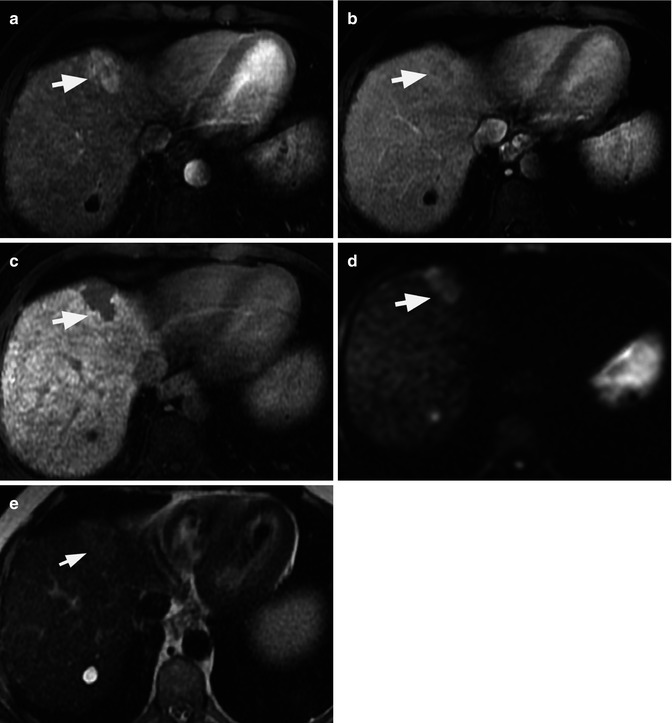

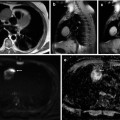
Fig. 40.4
Liver MRI of classic hepatocellular carcinoma (HCC) in segment VIII in cirrhotic liver (arrows) evaluated by gadoxetic acid. Relative to liver parenchyma, the HCC is mildly hyperenhancing on arterial phase image (a), hypointense on venous phase (washout) image (b), and hypointense on hepatobiliary phase image (c) obtained 20 min after gadoxetic acid injection. The lesion also demonstrates high signal intensity on DWI (b = 500 s/mm2). There is a simple cyst in segment VII of the liver as demonstrated by its high signal intensity on T2 sequence (e)
The clinical recommended dose of gadoxetic acid is 0.025 mmol/kg instead of the 0.1 mmol/kg that is the recommended dose for the other nonspecific extracellular gadolinium-based contrast agents. This low clinical dose of gadoxetic acid may lead to lower enhancement of focal liver hypervascular lesions and portal vein during the arterial and venous phases, respectively, as compared to nonspecific gadolinium, hampering the detection and characterization of HCC and the assessment of arterial anatomy and portal vein patency [43]. Moreover, the injection volume of gadoxetic acid is also smaller, resulting in a possible acquisition timing error and truncation artifacts in the arterial phase. Doubling the dose of gadoxetic acid has also been suggested, as the degree of focal hepatic lesion enhancement in the arterial and parenchymal liver uptake in HB phases is dose dependent. In a study of patients with cirrhosis and HCC, it has been shown that doubling the dose improves the tumor-to-liver contrast during the arterial phase in all patients and also during the HB phase [44]. This may be especially relevant in the subgroup of patients in whom the liver function impairment causes a lower liver uptake of liver-specific contrast agent on HB phase, such as in Child B or C cirrhotic patients. Nevertheless, the weak uptake in the HB phase may mask the lesions as they appear similar to background liver parenchyma, canceling the potential contribution of the HB phase over the lesion characterization [44, 45]. The clinical impact of this policy in terms of lesion detection and the cost-effective analysis had not been evaluated.
There is more limited experience in clinical practice using gadoxetic acid in comparison with extracellular nonspecific gadolinium. The former is well tolerated and has a rate of adverse events similar than those reported for nonspecific gadolinium chelates. Nevertheless, the bolus injection of gadoxetic acid had been associated to an acute self-limiting dyspnea occurring significantly more often than when intravenous unspecific extracellular gadolinium is used. The higher rate of this side effect results in a more frequent degradation of the quality of arterial phase, limiting the detection of arterial-enhancing nodules [46]. In order to avoid a higher rate of these artifacts, it is advisable to inject gadoxetic acid bolus at a rate not higher than 1 mL/s [47].
There is very limited data on MR elastography as a tool to characterize focal liver lesions. MR elastography is an imaging modality allowing direct visualization and quantitative measurement of propagating mechanical shear waves in biological tissue. The technique is used to obtain spatial maps (elastograms) and measurements of shear wave displacement. Preliminary data suggests that the technique can differentiate benign solid hepatic focal lesions (hemangiomas, focal nodular hyperplasia, adenoma) from malignant focal lesions (metastasis, cholangiocarcinoma, HCC), but the important overlap of stiffness values observed between different malignant lesions does not allow their proper characterization [48].
40.3 Diagnosis of HCC
40.3.1 Current Noninvasive Diagnostic Criteria
The most accepted criteria for noninvasive diagnosis of HCC, representing the current diagnosis criteria purposed by the American Association for the Study of Liver Diseases (AASLD) [6], defines that an HCC can be diagnosed by imaging when a nodule >1 cm in a cirrhotic liver displays arterial hypervascularity relative to liver parenchyma, followed by hypointensity in portal or delayed venous phase on CT scan or MRI. In such cases, tissue biopsy is not needed to confirm the diagnosis. These criteria had been prospectively validated in a few studies [3, 18, 30]. Although this typical vascular pattern can also be identified in some HCCs evaluated by CEUS, it is not specific for HCC as it had been described in a significant proportion of intrahepatic cholangiocarcinomas in cirrhotic patients [49, 50]. Therefore, neither the AASLD nor the European Association for the Study of the Liver (EASL) guidelines recommend the use of CEUS for the non-invasive diagnosis of HCC [6, 17].
40.3.2 Contribution of Functional Imaging Techniques over the Diagnosis of HCC
Although the AASLD criteria, based on morphological and conventional MRI findings, are highly specific for HCC (nearly 100 %), the main limitation is their low sensitivity especially for small HCCs that were reported at approximately 60 % with a negative predictive value below 50 %. Therefore, there is a clear need to improve sensitivity of diagnosis of HCC keeping a high specificity rate, and this might be possibly achieved by using additional or alternative MRI criteria. In a recent study by Park et al. [51]including 72 HCCs with transplant correlation, the sensitivity for the detection of HCC was not significantly improved after the addition of DWI imaging to standard gadolinium-enhanced imaging (59 % sensitivity without DWI vs. 63 % with DWI). Piana et al. [52] reported that the sensitivity for the diagnosis of HCC increased to 85 % when typical arterial hyperenhancement is followed by washout on the venous dynamic phase or hyperintensity on DWI compared to the sensitivity of the AASLD criteria alone (60 %). However, this study did not report the specificity of these alternative criteria, representing a major impediment to use them for the noninvasive diagnosis of HCC at this time [52].
As for the diagnostic criteria for HCC using gadoxetic acid, arterial hyperenhancement followed by portal-venous washout is the most accepted criteria. Although most typical HCC also display HB hypointensity, this feature cannot replace the classical washout seen in portal-venous or delayed phases for the diagnosis of HCC since this may reduce the specificity in the characterization of focal liver lesions as other tumors, such as cholangiocarcinoma, may display similar features [53]. However, it has been reported recently that in atypical nodules in cirrhotic liver, the identification of hypointensity on HB phase alone can be used as the strongest parameter for determining the diagnosis of HCC in cirrhotic liver [54, 55]. However, more data should be obtained about the specificity of the HB hypointensity sign in prospective studies that should include patients with other nodules than HCC and dysplastic nodules. Meanwhile, a non-hypervascular nodule in the arterial phase and hypointense in the HB phase of gadoxetic acid should be regarded as intermediate risk, whereas one that is hypervascular in the arterial phase should be regarded as a high-risk lesion [35, 54–57].
The combination of diffusion features, dynamic profile, and HB phase hypointensity offers the possibility to target three processes in hepatic carcinogenesis: hemodynamic changes, hepatocyte function, and tissue diffusivity. Recently, Kim et al. [58] reported that those atypical nodules for HCC with high SI on DWI (b = 800) and moderate to marked hyposignal intensity on the HB are at high risk to become typical hypervascular HCC during the follow-up (mean follow-up 388 days). Interestingly, those atypical nodules that progressed to HCC during the follow-up, presented at the initial MRI examination, significantly lower ADC values than those that do not progress to HCC.
The incidence of ICC has emerged in the last decade worldwide in cirrhotic and in non-cirrhotic population [59]. The systematic screening by US in cirrhotic patients had lead to detect small ICC in those patients [60]. The absence of contrast washout on extracellular gadolinium MRI could be helpful in the diagnosis of intrahepatic cholangiocarcinoma, but with the introduction of liver-specific contrast agents, some ICCs may display arterial hypervascularization with venous hypointensity mimicking the washout. This discrepancy can be explained by the peculiar characteristic of some liver-specific contrast agents (i.e., gadoxetic acid) as they can start to be taken in hepatocytes as early as 1 min after intravenous administration. The differentiation between HCC and ICC by imaging is very relevant because of its different treatment options and prognostic implications, but the final diagnosis of ICC usually relies on percutaneous biopsy as there is no specific imaging findings that allows its diagnosis. Recently, the combination of DWI sequences and gadoxetic acid findings had been used to demonstrate the differential features of ICC and HCCs. However, the target sign on HB phase is more frequently seen on large ICCs, and its reported sensitivity is low (32–43 %). Therefore, to obtain the final diagnosis of a nodule with atypical vascular pattern for HCC in a cirrhotic patient, histological confirmation is still needed for a proper differentiation between HCC and ICC [34].
40.4 Assessment of Treatment Efficacy
40.4.1 Response of HCC After Locoregional Therapy
The accurate evaluation of response to treatment is a key aspect in cancer therapy, because objective response in HCC after treatment may become a surrogate marker of improved survival. Most patients with HCC in the early and intermediate stages undergo locoregional therapies, including ablation and transarterial chemoembolization (TACE). Traditionally, therapeutic response of solid tumors has been assessed by serial tumor burden measurements according to Response Evaluation Criteria in Solid Tumors (RECIST) or its more recent revision RECIST 1.1 [61, 62]. However, the applicability of RECIST criteria is far to be optimal in the setting of HCC as they disregard tumor necrosis due to treatment, which is the objective of all effective locoregional therapies widely used for HCC [8, 9].
Estimation of the reduction in viable tumor volume, recognized as non-enhanced areas using dynamic imaging techniques, should be considered the optimal method for assessing local response to treatment in patients with HCC, and this represents the basis of the EASL criteria. Residual disease or recurrence is defined as presence of enhancing areas inside or next to the treated lesions after locoregional treatment [63]. RECIST criteria missed all complete response and underestimated the extent of partial tumor response due to necrosis. Therefore, EASL criteria is a better surrogate marker in predicting clinical response and survival than tumor shrinkage (Fig. 40.5) [8, 9]. However, despite this, the results reported in the literature are somewhat heterogeneous using CT and/or MRI for detecting residual tumor after locoregional therapies compared with liver explants [8, 64, 65], with a sensitivity ranging from 36 to 87 % and specificity of 63–100 %. This variability of results can be secondary to technical details and differences in the interval time between imaging and liver transplantation. Also, CEUS in expert hands may represent a reliable alternative to assess the therapeutic response after HCC ablation during the initial follow-up [7].
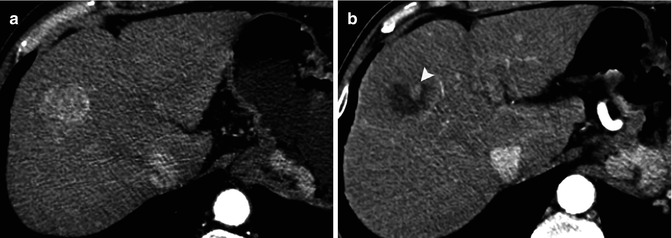

Fig. 40.5
Computed tomography of a cirrhotic liver with a hepatocellular carcinoma (a) in the right hepatic lobe. After transarterial embolization, a residual tumoral area is seen as enhancing nodular area inside the necrotic cavity (arrow in b)
Recently, RECIST criteria were adapted for HCC in order to refine both RECIST and EASL criteria, and as per its guidelines, a target lesion should meet the following criteria: the lesion should be classified as measurable (well-defined margins), should be suitable for repeated measurements, and must display intratumoral enhancement on contrast-enhanced CT or MRI. This is recognized as modified RECIST (mRECIST) and follows the same cutoff points as RECIST for defining the different categories of tumor response [66]. Although the introduction of mRECIST initially gained increased recognition as a potential tool, especially for clinical trials, it has not been widely validated [67]. Furthermore, the measurement of the diameter of residual viable tumor after ablation, TACE, or molecular therapies may be challenging in lesions showing partial response, especially those with larger diameter as they usually depict heterogeneous enhancement even before the treatment.
Only few authors have evaluated the potential contribution of DWI in the assessment of the response after the locoregional treatment. DWI potentially represents a helpful tool to differentiate inflammatory changes surrounding the necrotic cavity from truly residual tumor in initial follow-up, preventing the interpretation of false-positive results. However, DWI failed in improving the detection rate of viable tumor based on the morphological findings seen on T2-weighted sequences and gadolinium-enhanced MRI [68, 69].
From a theoretical point of view, findings obtained during the HB phase of liver-specific contrast agents may improve the specificity of MRI for detecting viable tumor, as it could be helpful for differentiating between the peritumoral inflammatory changes that can be seen at initial follow-up after locoregional therapy of HCC, from truly viable tumor. To our knowledge, there is scarce data in the literature evaluating the added value of HB phase for detecting viable tumor after ablation therapy over the conventional MRI sequences. Watanabe and cols [70] found that neither the specificity nor the sensitivity of MRI for the detection of local recurrence was improved by including HB phase on a series of 61 HCCs after ablation.
40.4.2 Response of Advanced HCC After Systemic Treatment
Sorafenib is currently the only systemic treatment that improves the survival of patients with advanced HCC [11, 12




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree