Ahmed et al. [36]
El Fegoun et al. [37]
Muto 2012
n
20
12
42
Age, mean (Y)
60.4
70
71
Pre-PSA, mean (range) (ng/ml)
7.3 (3.4–11.8)
7.5 (2.6–10.0)
5.8 (3.8–20.0)
Follow-up periods
12 months
Median, 10 years
Median, 62 months
Gleason sum  6
6
 6
6Low risk, 5 (25%)
10 (83%)
29 (69%)
7
Intermediate risk, 15 (75%)
2 (17%)
6 (14%)
 8
80
0
5 (11%)
PBx positive rate at 1-year
10.5%
9%
18.4%
Table 20.2
Adverse effects after focal HIFU: very few patients suffered from adverse effects
Ahmed et al. [36] | El Fegoun et al. [37] | Muto 2012 | |
|---|---|---|---|
N | 20 | 12 | 42 |
Pad-free urinary continence | 18 (90%) | 12 (100%) | 42 (100%) |
Urinary retention | 0 | 1 (8.3%) | 1 (2.4%) |
Urethral stricture | 1 (5.0%) | 0 | 1 (2.4%) |
Rectourethral fistula | 0 | 0 | 0 |
Urinary tract infection | – | 2 (16.7%) | 1 (2.4%) |
Focal High Intensity Focused Ultrasound at Teikyo University Hospital
We use the latest generation Sonablate® 500 which has a combined therapy imaging transducer. This device is able to use two focal lengths (30 and 40 mm) to increase the resolution of the treatment plan and precise control of energy delivery by each pulse. Our targeted area of FT is posterior three-fourth ablation (Fig. 20.1). It is the hemiablation plus posterior contralateral region which covers bilateral peripheral zone. Our inclusion criteria of FT were as follows: cancers that confined to one lobe judged by digital rectal examination (DRE), systematic biopsies (PBx) of more than 12 cores and magnetic resonance spectroscopy (1H-MRS) and imaging, PSA less or equal 20, Gleason score less or equal 8, no limit on number cores positive, and no limit on cancer core length. Follow-up criteria are DRE and PSA for every 3 months and 1H-MRS and routine biopsy at 6 and 12 months after focal HIFU.
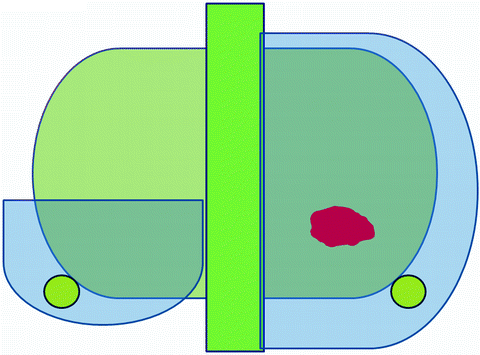

Fig. 20.1
Our targeted area of FT is posterior three-fourth ablation. It is the hemiablation plus posterior contralateral region which covers bilateral peripheral zone
A total of 42 patients received focal HIFU. The mean age was 71 years. The number of patients with hormone therapy (LHRH analogue monotherapy) immediately before focal HIFU was only two. The median preoperative PSA level was 5.8 ng/mL. Median numbers of positive cores were 2. Preoperative T stages were T1c and T2a. Although the most common Gleason score is 3+3, some patients with Gleason grade of more than 4 were included. Median follow-up periods were 62 months (Table 20.1).
After focal HIFU, PSA levels constantly decreased over a long duration of up to 72 months (Fig. 20.2). Overall biochemical disease-free 5-year survival according to Phoenix criteria was 53.3% and median survival time was 48.7 month. Pathological disease-free survival according to scheduled PBx was 88.1% at 6 months and 81.6% at 12 months after focal HIFU (Table 20.1).
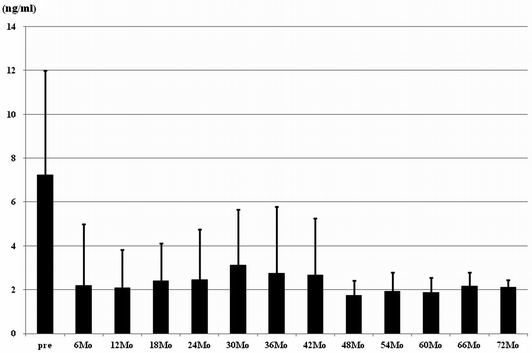

Fig. 20.2
Sequela of serum PSA level after focal HIFU at Teikyo University
When compared with the patients in whole therapy group, the period of indwelling urethral catheter after HIFU was significantly decreased in FT group (15.2 ± 4.4 vs. 19.7 ± 7.6 days, P = 0.0213). Frequency of urethral stricture and symptomatic urinary tract infection tended to be higher in whole therapy group (3/35: 8.6%, 4/35: 11.4%, respectively) as compared to the patients in focal therapy group (1/42: 2.4%, 1/42: 2.4%, respectively), although statistically nonsignificant (Table 20.2). These results indicate that focal HIFU causes fewer side effects when compared with whole HIFU.
Who Is the Appropriate Candidate for Focal HIFU?
As referred to above, the oncological outcome of our focal HIFU was not acceptable.
What is the cause of the disastrous results? This study included the patients with high PSA level of 20 ng/ml and high Gleason grade of more than 4 (Table 20.1). It seems very possible that the results of these patients with high risk certainly affect the poor outcome. One of the most important clinical practical problem points is the appropriate concrete criteria for FT. Although some clinical criteria were recommended for FT previously, it had not yet been established. One of these is the recommendation from The International Task Force on Prostate Cancer and Focal Lesion Paradigm [23]; Clinical criteria: cT1 or cT2a [38], PSA <10 ng/ml [24, 39], PSA density<0.15 ng/ml/cc [40], PSA velocity <2 ng/ml/year [39], Biopsy criteria: ≥12cores [41], Gleason grade <4 [38], % of cancer in each core < e.g., 20% [42], length of cancer in each core < e.g., 7 mm [43], % of total cores with cancer < e.g., 33% [44], Imaging criteria: single lesion < e.g., 12 mm, length of capsular content < e.g., 10 mm, no evidence of extraprostatic extension or seminal vesicle invasion. We examined the validity of the Task Force inclusion criteria in cases of our focal HIFU. Therefore, our patients were categorized into two clinical subgroups, patients who met the Task Force Criteria and the others who did not. I compared the oncological outcome between two groups. Twenty-three patients met the Task Force Criteria. There were significant differences in PSA values between the two groups at 3, 6, and 9 months after focal HIFU (Fig. 20.3). There were significant differences in 5-year biochemical disease-free survival rate between the two groups (χ 2 = 8.0487, P = 0.0046) (Fig. 20.4). Five-year biochemical disease-free survival rate for patients meeting the Task Force Criteria was 78.5% (median survival 64.8 months). On the other hand, the rate for patients not meeting the Task Force Criteria was 28.4% (median survival 32.8 months). There were significant differences in pathological proven recurrence rate between the two groups (χ 2 = 8.0498, P = 0.0046). Of the 19 patients not meeting the Task Force Criteria, only 10 patients had negative biopsy findings (52.6%). On the other hand, of the 23 patients meeting the Task Force Criteria, 21 patients had positive biopsy findings (91.3%). Consequently, the recommendation from The International Task Force on Prostate Cancer and Focal Lesion Paradigm [23] seems to be valid criteria for focal HIFU. But, some other several variables and other pathological factors may be typically used to judge patients to be a candidate for FT. MRI has shown promise as a means to distinguish between clinically insignificant and significant prostate cancer [45]. We should estimate the effectiveness of many other inclusion criteria for focal HIFU including imaging methods as well.
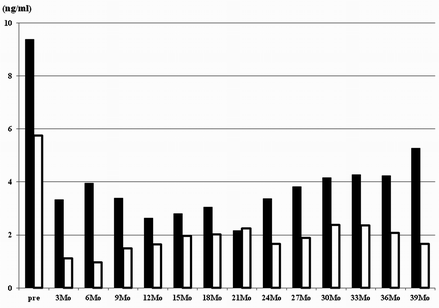
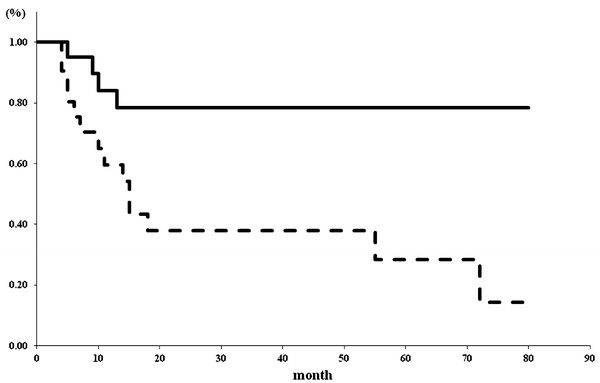

Fig. 20.3
Oncological outcome between two clinical subgroups: patients who met the Task Force Criteria and those who did not. White box indicated the patients meeting the Task Force Criteria (n = 23). Black box indicated the patients not meeting the Task Force Criteria (n = 19). There were significant differences in PSA values between the two groups at 3, 6, and 9 months after focal HIFU

Fig. 20.4
Five-year biochemical disease-free survival rate for the patients meeting the Task Force Criteria was significantly higher (78.5%, median survival 64.8 months) when compared with the patients not meeting the Task Force Criteria (28.4%, median survival 32.8 months) (χ 2 = 8.0487, P = 0.0046). Solid line indicated the patients meeting the Task Force Criteria (n = 23). Dotted line indicated the patients not meeting the Task Force Criteria (n = 19)
Challenges for the Future
What need to be done to let FT standard? What is the “Focal” therapy? We must confirm the definition of FT as soon as possible. We should clear not only terminological differences but also practical and scientific differences between “Focal” and “Partial” therapy. What is the therapeutic main objective for FT, radical treatment or QOL? The objective is closely associated with the definition and clinical role of FT to localized prostate cancer. For practical clinical use, we should standardize FT protocols: patient selection criteria, patient exclusion criteria, treatment planning including with or without concomitant hormonal therapy [28], defining outcomes, follow-up schedule, follow-up criteria, and secondary therapy after FT.
Although it is clear that hormone therapy has benefits when used in combination with radiation therapy for locally advanced disease [46], and when added to prostatectomy in men with nodal metastases, it is not clear when used for FT, especially focal HIFU.
With respect to criteria for recurrence, although some authors have reported that a high PSA nadir value correlates with HIFU failure [47], we have no sufficient data about the effectiveness of PSA nadir after Focal HIFU. Also, it is still debatable if PSA is the adequate measure to define the recurrence. It is well known that several imaging techniques such as CT and MRI are less successful in making diagnosis of recurrence after treatments such as radiation therapy, HIFU, and cryosurgery. Especially, in HIFU, tissue heating induces coagulative necrosis in the target area, which becomes completely devascularized and surrounded by inflammation and edema [48, 49]. MR images obtained within days after HIFU show a significant increase in prostate volume, presumably due to transient edema, with slightly hyperintense areas on T1-weighted images, most likely representing interstitial hemorrhage, and a central hypointense and ill-defined lesion on T2-weighted images [50]. After 5 months, the prostate shrinks and the parenchyma becomes diffusely hypointense and ill-defined with loss of the normal zonal anatomy on T2-weighted images [50]. Although residual and recurrent malignant tumors of the prostate have been reported to be somewhat more hypointense than the surrounding tissue, it remains difficult to correctly discriminate the two entities with anatomic T2-weighted MRI alone [51]. In sum, at the present, no definite image studies are available routinely to circumvent biopsy to know the exact localization of the prostate cancer. However when modalities to identify the localization of prostate cancer developed after HIFU, focal HIFU would possibly be a feasible way of choice in the treatment of small-volume prostate cancer. We require further development of imaging devices such as 3D multivoxel spectroscopy [52] to improve imaging methods in sensitivity of local recurrence after Focal HIFU.
Additional studies and long-term follow-up are needed to confirm that Focal HIFU could have a profound effect on prostate cancer management.