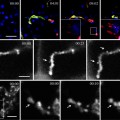
Fig. 1.
Double labeling of LDs and adipocyte differentiation-related protein (ADRP) in Huh7 cells. (a) Cells were immunolabeled for ADRP after fixation using a Cy3-conjugated secondary antibody and then stained for LDs by BODIPY493/503. ADRP (red) is observed around spherical LDs (green). (b) Cells were cultured with BODIPY558/563 C12, fixed and immunolabeled for ADRP using an Alexa488-conjugated secondary antibody. ADRP (green) and LDs (red) are seen in the same manner as in (a) but in opposite colors. Scale bars, 10 μm.
3.1.2 Nile Red
2.
Rinse with PBS three times.
3.
Mount on a glass slide with the Mowiol solution containing Nile red (see Note 5). Alternatively, incubate with the Nile red solution in PBS for at least 10 min in the dark and then rinse three times in PBS.
4.
Store specimens in a lightproof box until microscopic observation.
3.1.3 Oil Red O
1.
2.
Rinse three times with PBS.
3.
Treat with 1 mg/mL sodium borohydride in TBS for 10 min to quench autofluorescence caused by glutaraldehyde (see Note 8).
4.
Rinse three times with PBS.
5.
Incubate in 60% isopropanol for 2–3 min.
6.
Incubate with the Oil Red O solution for 30 min.
7.
Rinse three times with 60% isopropanol.
8.
Rinse three times with PBS.
10.
Observe under a fluorescence microscope with a filter set for G excitation (used for rhodamine, Cy3, Alexa568, or RFP).
3.2 Staining of LDs in Live Cells
3.2.1 BODIPY 558/568 C12
2.
Replace culture medium with fresh not containing the dye and incubate for 30 min.
3.
4.
Observe under a fluorescence microscope with a filter set for G excitation (used for rhodamine, Cy3, Alexa568, or RFP) (Fig. 1b). The excitation and emission wavelengths of BODIPY 558/568 C12 are 558 and 568 nm, respectively.
3.3 Immunolabeling of ADRP
2.
Rinse three times with PBS.
3.
Treat in 50 mM NH4Cl in PBS for 10 min to quench residual aldehydes.
4.
Rinse three times with PBS.
5.
Treat with 1 mg/mL sodium borohydride in TBS for 10 min to quench autofluorescence caused by glutaraldehyde (see Note 8).
6.
Rinse three times with PBS.
8.
Rinse three times with PBS.
9.
Treat in 3% BSA in PBS for 10 min to block nonspecific binding.
10.
Rinse three times with PBS.
11.
Incubate with mouse anti-ADRP antibody (diluted 1:5) in PBS containing 1% BSA for 30 min at 37°C.
12.
Rinse three times with 0.1% BSA in PBS for 5 min.