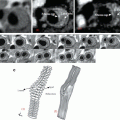
Histology
Carotid ultrasound
Major
Echolucency (low gray-scale median)
Thickness of the fibrous cap
Thickness of the echogenic cap
Large inflammatory cells infiltrate
Surface irregularity/ulceration
Superficial erosion or fissure
Proximity of the echolucent region to the lumen
Presence of thrombus
Severe stenosis
Size of the lipid core
Lesion motion
Severe stenosis
Others?
Minor
Superficial calcified nodule
Intraplaque hemorrhage
Endothelial dysfunction
Outward remodeling
Others
Rich in proteoglycans
Others?
Nomenclature has motivated many discussions and meetings. The use of terms like the unstable, high-risk, thrombosis-prone, symptomatic, disrupted, or vulnerable plaque has been somewhat confusing. The language can be misleading as plaques are not symptomatic as the patients are the ones who may have symptoms, but that term is supposed to be a simplification of “plaque associated with symptoms”. Unstable is not a very specific term, while the prospective definitions, such as vulnerable/high-risk/thrombosis-prone, seem more adequate. Independently of the nomenclature, one should aim at the identification of the plaques that have a high likelihood of causing clinical events. To this end, many different methods have been used and are still being developed and improved, from histological and biochemical methods to ultrasonographic and magnetic resonance imaging and others, such as elastography and thermography [50].
5 Ultrasound
Nowadays, the indications for carotid endarterectomy are based on the presence of symptoms and on the degree of stenosis. However, atherosclerotic plaques causing low grades of stenosis have been associated with clinical events, while others causing high degrees of stenosis may remain asymptomatic [51, 52]. Because of this, the degree of luminal narrowing may not be the optimal criterion for defining clinical risk and indications for treatment. As a result, some patients may undergo unnecessary surgery. Improved techniques are, therefore, needed to enable reliable identification of high-risk plaques that lead to cerebrovascular events. In the last decade, ultrasound has been further developed using compound imaging, [53, 54] and 3D reconstructions [55, 56].
Carotid ultrasonography has known limitations in discriminating subtotal from total occlusion, in assessing plaques that have an acoustic shadow, and in patients with short neck, high carotid bifurcation, or severe arterial kinking. Operator dependency has also been a limitation that has been discussed widely, but is beyond the scope of this chapter.
Newer generations of ultrasound equipment have appeared, as attempts to overcome some of the limitations. An example is the high-definition ultrasonography. It has higher spatial, contrast, and temporal resolution, allowing also direct digital image recording. The latest strategy in our group is to use open platform equipments and design homemade algorithms to characterize plaque morphology.
Several subjective classifications for the ultrasonographic aspect of plaques have been proposed: Reilly et al. [57] divided plaques into homogeneous and heterogeneous; Johnson et al. [58] into calcified, dense, or soft; Gray-Weale et al. [59] into echolucent, predominantly echolucent, and predominantly echogenic and echogenic ones; Langsfeld et al. [60] also graded as to the ratio of echolucency to echogenicity, with type 1 being most echolucent and type 4 being most echogenic, and the normal-appearing artery was classified as type 5; for Geroulakos et al. [61] the type 5 consisted of plaques that could not be classified owing to acoustic shadows; the European Carotid Plaque Study Group [62] divided plaques into echolucent, intermediate, and echorich. In summary these studies suggested that the soft, echolucent, and heterogeneous plaques were those associated with symptoms. In Geroulakos’ study the association of symptoms and echolucency was only true for stenosis >70%. Others could not find associations between the ultrasonographic aspect and neurologic events [63, 64]. Many of these studies were based on small cohorts of patients and the ultrasonographic evaluation was done subjectively.
Actually, the most important limitation of ultrasonography is the intra- and inter-observer variability. Standardization methods to process plaque images have been found to overcome differences in the evaluation of plaque echogenicity between the different equipments and observers. This way, the group from the Irvine Laboratory at the St. Mary’s Hospital, London [65, 66], created a method, based on the gray-scale values of pixels in a scale of gray intensities (0 darkest and 255 brightest). They used the gray-scale median (GSM), as a score of global echogenicity. The reference values were the blood as 0 (the darkest structure in the image) and the adventitia as 190 (the brightest structure in the image). The whole plaque image outlined would be processed by linear scaling, compressing the scale. Making the blood and adventitia of all the plaques have the same values allowed the comparison of different plaques, assessed with different equipments and setups and by different observers. Intra- and inter-observer variabilities improved considerably [65].
Plaques with low GSM have been associated with higher incidence of cerebral infarction [67, 68]. These authors found a cutoff value of 32 for GSM that could discriminate best between the plaques associated with symptoms or not. The same cutoff was identified by our group [69]. Other studies have found other cutsoff values of GSM, namely 40, using the same method [66], or 50, without using the linear scaling [70].
Gronholdt et al. [38] showed that echolucent plaques causing stenosis >50% are associated with increased risk of stroke in symptomatic but not asymptomatic individuals. Using GSM = 74 as a cutoff point, echolucency was an age-independent predictor of ischemic stroke in symptomatic patients. In symptomatic patients, relative risk of ipsilateral ischemic stroke for echolucent versus echorich plaques was 3.1. Based on the degree of stenosis, when comparing 80–99% versus 50–79% stenosis, the relative risk was 1.4. This association between echolucency and high risk for ipsilateral symptoms has also been verified on other studies, though the influence of the degree of stenosis is not widely accepted [71, 72]. Whereas some suggested hypertension and progressive lesions to be important additional determinants of risk [73], others proposed that echolucent plaques were associated with increased risk of neurological events, even independently of degree of stenosis and cardiovascular risk factors [74].
Some other studies evaluated heterogeneity of the plaque image and found that heterogeneous and/or echolucent plaques are associated with higher risk of stroke [60, 75–79]. The ability of ultrasonography to study other characteristics that could be associated with the presence of symptoms has been intensively studied. The identification of ulceration by ultrasonography in different studies showed large variation. Rubin et al. [39] using ultrasonography detected 93% of the ulcerated lesion, whereas Comerota et al. [80] argued that the possibility of detecting ulceration varied with different degrees of stenosis, being particularly difficult in high-grade stenoses. Bluth et al. [81] reported that merely sonography for evaluating the surface of the plaque to determine if it was smooth or irregular could not be used as a successful means to identify which patients were at risk for ulceration. O’Leary et al. [82] came to a similar conclusion. Bassiouny et al. [83] found that the proximity of plaque necrotic core to the lumen is associated with clinical ischemic events. Our group [40] showed that in heterogeneous plaques, juxtaluminal location of the echolucent region was associated with increased risk. On the contrary, in homogenous plaques the absence of an echogenic cap and disruption of the plaque surface correlated with symptoms. In an attempt to determine the relative importance of the ultrasonographic structural characteristic of the plaques, besides GSM, an activity index was calculated. This activity index was associated with symptoms [84]. The parameters associated with the presence of ipsilateral symptoms were surface disruption, severe stenosis, and low GSM and, in heterogeneous plaques, the presence of a juxtaluminal echolucent area. Though all these results are interesting, large-scale studies on different ultrasonographic aspects that can reflect risk and on the natural history of the plaques are needed.
Although the association of echolucent plaques with higher neurological risk has become accepted, no ultrasonographic characteristics have been associated with a single type of symptom. The majority of the studies did not separate those end-points. One study, by Sabetai et al. [85], reported that plaques associated with amaurosis fugax are more hypoechoic and more stenotic than those associated with TIA or stroke or those without symptoms. In another study by Iannuzi et al. [86] TIA patients showed more hypoechoic carotid plaques with longitudinal motion. The association between plaque radial and longitudinal motion and neurological events was demonstrated by four-dimensional ultrasound examinations in another study [41].
In order to better understand the ultrasonographic appearance of plaques, histological analyses have been used in several studies. Wolverson et al. [87] compared echolucent and echogenic plaques in vitro, relatively to their composition. Aggregates of amorphous lipid residue appeared less echogenic than adjacent tissues and regions of dense fibrosis more echogenic. Densely calcified foci in plaques were highly echogenic and associated with acoustic shadowing. Gray Weale et al. [59] when creating the classification of ultrasound appearance in 1988 observed the plaques macroscopically and histologically. There was a significant relationship between plaques type 1 and 2 (the echolucent and predominantly echolucent) and the presence of either intraplaque hemorrhage or ulceration. Montauban van Swijndregt et al. [88] concluded in a first study that B-mode ultrasound and subsequent subjective categorization of atherosclerotic plaques cannot adequately determine the volume of fibrosis or lipids within the plaque. Later [89] they found that plaques associated with symptoms contained more fibrosis than lipids. Apart from agreeing that echolucent plaques were most common in symptomatic patients Kardoulas et al. [90] reported that the fibrous tissue was significantly greater in echogenic plaques. According to Lammie et al. [91] echolucent areas in the plaque corresponded histologically to necrosis or hemorrhage, and the thickness of the fibrous cap could be determined reliably with ultrasound. On the other hand a possible association between clinical events and the presence of hemorrhage in the plaques has not been consensual. Some supported that association [17, 18], while others did not [92, 93]. Similarly, the ability of ultrasound to detect plaques with hemorrhage is also not consensual. Actually in most ultrasound studies comparing with histology, the criteria to define plaque hemorrhage varied or no distinction was made between lipid and hemorrhage. They have often been called “soft tissue” [62]. Nevertheless, plaques with a high lipid and hemorrhage content as established histologically had low GSM, whereas those with a high fibrous content had a high GSM [94]. According to Gronholdt et al. echolucent carotid artery plaques were associated with elevated levels of acute phase reactants, [95] and circulating triglyceride-rich lipoproteins in the fasting or postprandial state [96]. Some years later, they reported that echolucency was associated with increased macrophage density and lipid content in the plaques [97]. Histologically, echogenic plaques contained more calcification and fibrous tissue than echolucent plaques. Intraplaque hemorrhage was directly related to lipid content and inversely related to amount of fibrous tissue in the plaque [98].
In many of the previous studies, the histological analysis was done only by semiquantitative or not quantitative techniques, using unspecific stainings and analyzing only parts of the plaques. Moreover, many of them only used the subjective classifications for the ultrasound images. Analyzing plaque components by other methods, such as biochemical methods in relation to echogenicity, has been done by our group, allowing the measurement of components in the whole plaque and not only on some sections. We used fast-performance liquid chromatography and high-performance thin-layer chromatography to measure matrix and lipid components in plaque homogenates [99]. Echolucent plaques contained less hydroxyapatite (the most prevalent calcium salt in plaques), more total elastin, and more intermediate-size elastin forms. There was no difference in collagen amount between echogenic and echolucent plaques, neither biochemically nor histologically. Cholesterol esters, unesterified cholesterol, and triglycerides were increased in plaques associated with symptoms, but no differences were detected between echolucent and echogenic plaques. Similar results were obtained by Oil Red O staining (for lipids) in symptomatic versus asymptomatic and in echolucent versus echogenic plaques. Our study was the first to study elastin and its fragments (known to be chemoattractant) in relation to echogenicity. Echogenicity seemed to be mainly determined by their elastin and calcium but not collagen or lipid content.
As inflammatory cells are so relevant in the atherosclerotic process, we also studied the cellularity in relation to echogenicity. Echolucency of carotid plaques correlates with plaque cellularity [100]. More recently it was found that annexin A1, a calcium and phospholipid-binding protein, known as endogenous modulator of inflammation, correlates with GSM in high-grade carotid stenosis [101]. This reinforces the idea of complex regulatory mechanisms of inflammation, which might be reflected in the ultrasound aspects of the plaques. Inflammatory cells secrete proteinases such as the metalloproteinases (MMP). Turu et al. [102] showed that hypoechogenic plaques had increased MMP activity and asymptomatic patients with plaque progression showed increase intraplaque MMP-8 levels.
Vascular calcification is a frequent process in the advanced lesions. Osteoprotegerin is one of the proteins involved in the regulation of bone metabolism and vascular calcification and high serum values of osteoprotegerin are associated with cardiovascular disease in humans. Vik et al. [103] showed that patients with echogenic carotid plaques had lower levels of serum osteoprotegerin, supporting its role in arterial calcification.
Intraplaque neovascularization is a common characteristic of advanced lesions. Feinstein’s group used contrast-enhanced ultrasound to visualize neovessels in the plaque and correlated that to the lesion severity and morphologic features of plaque instability [104]. An additional concept attempted by Owen et al. has been to measure the retention of nontargeted microbubbles using contrast-enhanced ultrasound to depict difference in plaques from asymptomatics or symptomatics [105].
The rate of remodeling of human plaque tissue has not been studied. We were the pioneers in measuring for the first time in humans the biological age of different components of advanced atherosclerotic plaques by analyzing tissue levels of 14C. The turnover time of human plaque tissue is very long (20 years or more) [106]. This may explain why regression of atherosclerotic plaque size rarely is observed in cardiovascular intervention trials. Simultaneously it recalls the interest of early lesion monitoring for instance on the fatty streak level. At that early biological phase, a possible intima–media thickening will be seen that would correspond to small accumulation of lipids and inflammatory cells in the intima. Recently, inspired by the measurements of GSM performed in advanced lesions, Lind et al. started to measure the GSM on the intima–media complex. Considering the fact that echolucency may correspond to lipid accumulation it was easy to understand their results showing that low shear stress in the common carotid is associated with a thick intima–media and with an echolucent intima–media complex. Furthermore, they found that echolucent carotid intima–media complex was a predictor of all-cause mortality after age 75 years [107]. In another large study, they found that the GSM of the intima–media of the common carotid artery is closely related to the echogenicity in over carotid plaques [108].
6 Possible Future Trends
In the future one would like to overcome some of the current limitations of ultrasound, such as resolution, different tissue characterization, three-dimensional reconstruction, or maybe combining algorithms of different characteristics to be able to detect the plaques that are associated with higher risk for rupture and consequent symptoms. Targeted imaging could also be an approach, although there is no consensus on what is/are the best target/s yet. Some techniques like optical imaging have started to implement this targeting to image proteases [109]. There have been attempts on direct tissue characterization based on the integrated backscatter showing that the index in fatty/necrotic atheromatous sites was lower than that in fibrous or calcified sites and the same as that in intraplaque hemorrhagic sites [110].
Plaque morphology assessed by ultrasound will potentially give insights into the disease and why some patients have higher risk than others. One example of this type of study was published by Östling et al.,[111] showing that type 2 diabetics have more echolucent moderate plaques than nondiabetics. Plaque size and echogenicity are related to different cardiovascular risk factors in the elderly [112]. Further developments in ultrasound will definitely improve risk stratification.
A final important use of carotid plaque ultrasound is to monitor the effect of interventions, considering the possible effects on plaque composition that lay behind those interventions. Several studies have shown the favorable effect of statins on echogenicity [113]. More recently even other interventions have been studied such as the illustrative study showing the association of low-dose metoprolol CR/XL with increased plaque echogenicity [114].
References
1.
Aschoff L (1930) Die arteriosklerose. Mediz Klinik (suppl 1):1–20
2.
Nordestgaard BG (1996) The vascular endothelial barrier – selective retention of lipoproteins. Curr Opin Lipidol 7(5):269–273PubMed
3.
Palinski W, Hörkkö S, Miller E, Steinbrecher UP, Powell HC, Curtiss LK et al (1996) Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest 98(3):800–814PubMed
4.
Palinski W, Rosenfeld ME, Ylä-Herttuala S, Gurtner GC, Socher SS, Butler SW et al (1989) Low density lipoprotein undergoes modification in vivo. Proc Natl Acad Sci USA 86:1372–1376PubMed
5.
Seifert PS, Hugo F, Hansson GK, Bhakdi S (1989) Prelesional complement activation in experimental atherosclerosis. Terminal C5b-9 complement deposition coincides with cholesterol accumulation in the aortic intima of hypercholesterolemic rabbits. Lab Invest 60(6):747–754PubMed
6.
Walpola PL, Gotlieb AI, Cybulsky MI, Langille BL (1995) Expression of ICAM-1 and VCAM-1 and monocyte adherence in arteries exposed to altered shear stress. Arterioscler Thromb Vasc Biol 15(1):2–10PubMed
7.
Brown MS, Goldstein JL (1983) Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem 52:223–261 [Review]PubMed
8.
Lehr HA, Seemuller J, Hubner C, Menger MD, Messmer K (1993) Oxidized LDL-induced leukocyte/endothelium interaction in vivo involves the receptor for platelet-activating factor. Arterioscler Thromb 13(7):1013–1018PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree