Fig. 24.1
High-speed photographic images of transient (inertial) cavitation activity in water illustrating the initiation, growth, coalescence, and collapse of microbubbles within 2 ms
Delivery of intense continuous ultrasound energy to produce both thermal and cavitational effects, known as pyrotherapy, was attempted several decades ago [14] but was ultimately abandoned as cavitation was deemed unpredictable and difficult to control [9, 10]. As a result, contemporary therapeutic ultrasound research has since primarily focused on production of thermal effects below the cavitational threshold [6–8, 15–17].
However, our research group at the University of Michigan has demonstrated that cavitation can be induced and controlled in tissue by delivering short (<50 μs), intense ultrasound pulses at a low duty cycle to minimize thermal effects and isolate cavitational activity [18–22]. Each ultrasound pulse creates a localized, highly dynamic cluster of microbubbles (a bubble cloud). The formation, oscillation, and collapse of microbubbles create localized stresses and pressures at the cellular and subcellular level. This form of therapeutic ultrasound has been named “histotripsy” to emphasize the nonthermal cavitational processes that produce mechanical tissue ablation. The appearance of the targeted tissue after histotripsy (Fig. 24.2) is distinct from thermal coagulative necrosis seen with HIFU. Tissue architecture and cellular structures are unrecognizable within the targeted zone consistent with mechanical homogenization [23].
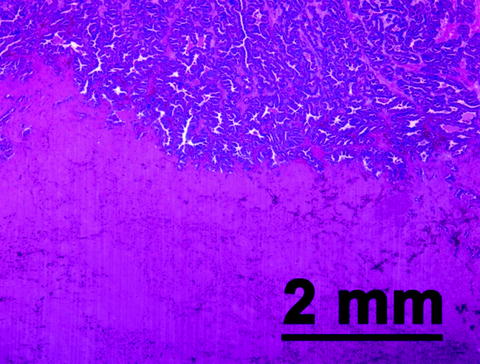

Fig. 24.2
A representative hematoxylin- and eosin (H&E)-stained slide of prostate tissue harvested shortly after extracorporeal histotripsy treatment in an in vivo canine model. Structurally intact prostate glandular tissue is seen in the top right portion of the slide. The bottom left aspect of the slide corresponds to the histotripsy target zone. Within this treated area, no recognizable cellular or architectural features are evident
Tissue Effects: Pulse Dependence
The process of microbubble formation, oscillation, and collapse as observed with histotripsy is a stochastic process. Bubbles develop at nucleation sites which are randomly distributed within the focal volume (i.e., the zone where the rarefactive pressure is sufficiently negative to induce bubble formation and growth). Cavitation and bubble collapse produce local zones of tissue homogenization. With application of additional pulses, these zones of damage enlarge and ultimately coalesce to cover the entire focal volume.
Experimentally this was seen in an in vivo rabbit model where histotripsy was applied to single focal volumes (3 × 3 × 10 mm) located within normal renal cortical tissue. Within each kidney, 10, 100, 1,000, or 10,000 pulses (20 μs) of ultrasound energy were delivered to each targeted volume from a 750-kHz piezoelectric transducer [2]. Delivery of 10 pulses produced scattered focal hemorrhagic zones within a 3 × 10-mm ellipsoid region corresponding to the targeted zone with multiple hemorrhagic zones containing some cellular debris (Fig. 24.3). Immediately surrounding each focal hemorrhage, a zone of architecturally intact though mortally injured cells with absent or pyknotic nuclei were noted. This zone spanned a distance of approximately 100 μm. Beyond this zone, a ring of less severe cellular damage was present, also spanning approximately 100 μm. When 100 pulses were delivered, a greater number of hemorrhagic areas of larger size were noted within the same-sized ellipsoid region. Surrounding each area of hemorrhage, the same two-tiered pattern of cellular injury was noted, with each ring again spanning 100 μm [2].
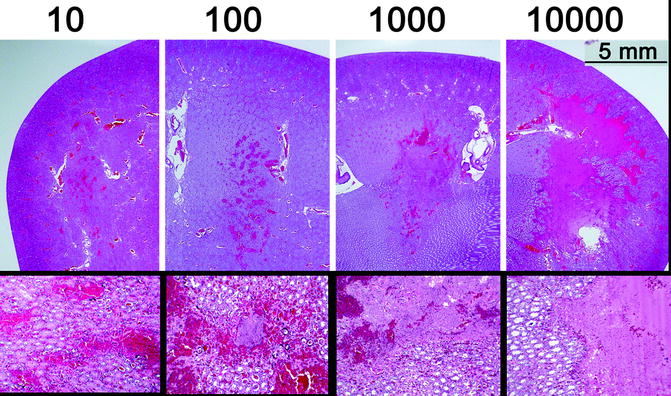

Fig. 24.3
Rabbit kidneys treated in vivo demonstrate the evolution of bioeffect with increasing pulses (top row) within the focal volume. Zones of focal hemorrhage after 10 pulses evolve to hemorrhage and tissue fractionation at 100 pulses, then coalesce with other foci and expand to encompass the majority of the focal volume after 10 k pulses. High-power images reveal the acellular debris present within the focal volume after treatment with a corresponding number of histotripsy pulses
After 1,000 and 10,000 pulses, an elliptical cavity with smooth boundaries and a liquefied core was apparent, corresponding to the dimensions of the focal volume (Fig. 24.3). Histological analysis was notable for large confluent areas of hemorrhage and acellular material thought to represent cytoplasm and fractionated cellular material. The few islands of recognizable parenchymal structure within the large confluent areas contained only mortally damaged cells. The same pattern of damage, ringing these large acellular regions, was evident as seen in the treatments with 10 or 100 pulses. With 10,000 pulses, the boundary of the confluent acellular region appeared to be more uniform with a narrower zone of peripheral damage. Interestingly, delivery of additional pulses further subdivided the material within the targeted zone but did not appear to enlarge the treatment zone [2].
Nonthermal Homogenization
The appearance of tissue treated with histotripsy is visibly and histologically distinct from tissue ablated with thermal modalities. This was demonstrated with ex vivo porcine kidneys submerged in degassed water. Thermocouples placed at target points within the cortex of the kidney were co-localized with the histotripsy ultrasound focus. Treatment consisted of sequential fractionation of a 3 × 3 grid of target points within the tissue, encompassing the position of the thermocouple tip. A range of parameters (intensity, pulse repetition frequency, number of pulses per point, duty cycle) was tested in this study. After treatment, the kidneys were sectioned, and treated target volumes were classified into categories (disrupted, desiccated, or mixed) based on gross appearance [18].
Interestingly, treated volumes characterized as disrupted (where liquefied material spilled out of a cavity after sectioning) were associated with a temperature rise no greater than 27 °C, indicative of nonthermal cavitational tissue destruction. Temperature increases of 40 °C or greater were associated with lesions classified as desiccated and consistent with thermal coagulative necrosis. The parameter sets when plotted in parameter space clustered based on the morphological appearance of the lesion produced (Fig. 24.4). Furthermore, these results confirm that ultrasound parameters can be selected to produce mechanical effects with negligible thermal contribution [18].
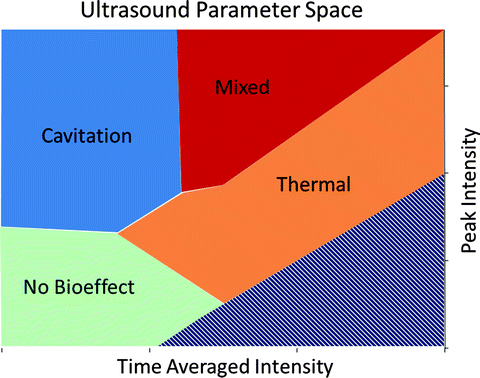

Fig. 24.4
Application of acoustic energy to in vitro porcine kidneys with varying parameters (time-averaged intensity and peak intensity) produced different tissue morphologies (disrupted, mixed, desiccated, and no effect). These morphologies clustered in parameter space and allowed creation of a plot with definable regions (cavitation, mixed, thermal, and no bioeffect)
Precision
The pattern of tissue destruction with histotripsy can be much more tightly controlled than with thermal ablative modalities. This is demonstrated in Fig. 24.5, where the histotripsy focus was scanned through a targeted volume, producing complete fractionation. In this case, the targeted volume was a cube, and the prostate was harvested 21 days after treatment. The precision of histotripsy is apparent when considering the straight lines and right-angle corners created and allows for “sculpting” of tissue. Within the prostate, this precision could facilitate prostate tissue homogenization without injury to critical periprostatic structures (neurovascular bundles, urinary sphincter, and rectum).
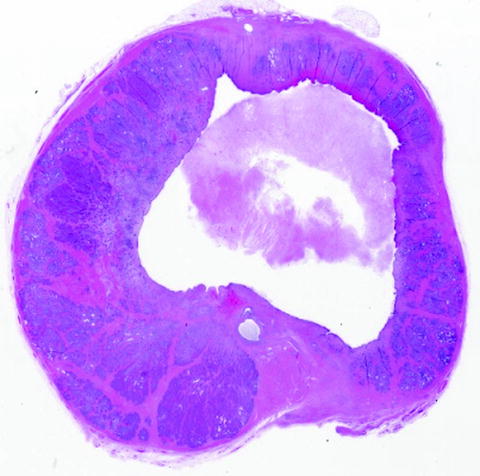

Fig. 24.5
H&E-stained sections of a canine prostate treated in vivo with histotripsy 21 days before harvest. The histotripsy focal volume was scanned through a 1 × 1 × 2-cm volume, encompassing the prostatic urethra. Although some collapse of the cavity has occurred, straight lines and sharp corners are apparent and indicative of the precision with which histotripsy can be applied
Real-Time Feedback
Histotripsy-induced tissue fractionation results from the production of microbubbles and their subsequent interaction with tissue. These same microbubbles are excellent sound reflectors and appear as hyperechoic (bright) zones on an ultrasound image [2, 12]. They can also serve as an inherent feedback mechanism for reliable and accurate target localization and real-time monitoring of treatment progression with high temporal (frame rate ∼100 Hz) and spatial resolution (Fig. 24.6). High-quality imaging feedback of the ablative process is essential for image-guided noninvasive techniques and is difficult to achieve with any current thermal ablation modality.
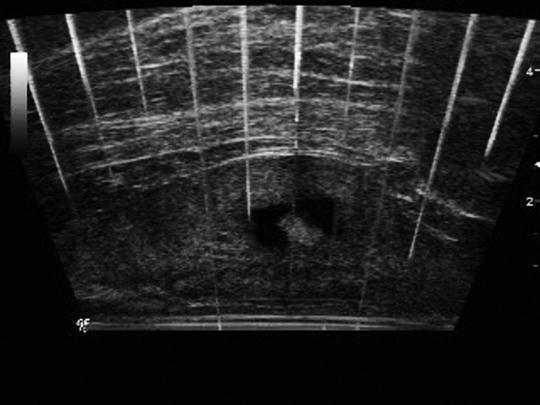

Fig. 24.6
Transrectal sagittal ultrasound image captured during histotripsy treatment of an in vivo canine prostate. The histotripsy bubble cloud (bright) is clearly visible within a treated volume of prostate tissue (dark rectangle). This form of real-time imaging conveys feedback of bubble cloud location and adequacy of treatment and provides a means to observe change in ultrasound character of tissue with progressive fractionation
During histotripsy treatment, the microbubbles respond to acoustic energy delivery by moving, oscillating in size, and changing density. This activity produces a temporally changing (twinkling) hyperechoic zone on an ultrasound image. Generally, histotripsy is performed with low duty cycle (0.1–1%). This allows observation of high-quality real-time B-mode ultrasound images during treatment with only occasional scan line corruption produced by large echoes from the therapeutic ultrasound pulses [24]. Following histotripsy treatment, fractionated tissue appears hypoechoic. Other characteristics of ultrasound backscatter data have also demonstrated utility as indicators of degree of tissue fractionation [25, 26].
Chronic Tissue Response
The tissue effect achieved with histotripsy, namely, cellular and structural homogenization, is different than the coagulative necrosis commonly seen with other ablative modalities. The biological response to this type of cellular destruction also appears different. In a chronic rabbit model, the left kidneys of 29 rabbits were treated with histotripsy and then harvested 1–60 days after treatment (Fig. 24.7). Grossly observable effects included a disrupted and hemorrhagic region on days 0–2, tubular dilation on days 7–60, and surface contour defects suggestive of lesion contraction on days 21–60. By day 60, there was near complete absence of scar formation, suggesting the acellular material generated by histotripsy appears to be rapidly reabsorbed within 45–60 days [27]. This compares favorably to results following ablation by thermal means. Following renal radiofrequency ablation, treated tissue did not reabsorb or shrink when monitored for 2 years after ablation in a study comparing radiofrequency ablation and cryoablation of benign and malignant renal masses in humans [28]. Following cryoablation, only 32% of ablated zones had shrunk to a radiographically undetectable size on follow-up imaging at the 2-year mark [28].
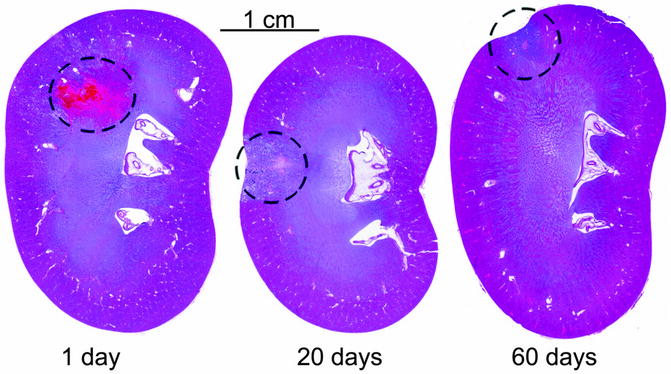

Fig. 24.7
Histological coronal sections of H&E-stained rabbit kidneys at 1, 20, and 60 days after histotripsy treatment of cortical tissue. Over time, resorption of the acellular homogenate occurs with minimal scarring. A contour defect forms at the margin overlying the treatment volume indicative of tissue volume loss
The liquid consistency of the tissue after histotripsy treatment has been seen to drain via urethra with prostate histotripsy when applied for tissue debulking (Fig. 24.8) as would be desired for BPH therapy [23, 29, 30]. For treatment of multiple foci of cancer within the prostate or an entire region of prostate, facilitated resorption or drainage may provide an advantage with respect to assessment of treatment adequacy and future surveillance.
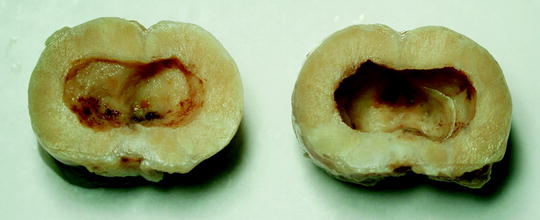

Fig. 24.8
Gross fixed histology (two halves sectioned transversely) of a canine prostate harvested 28 days after histotripsy treatment. The objective of treatment in this experiment was to debulk the central portion of the prostate. Homogenized material drained via urethra after treatment, leaving a smooth-walled cavity without internal debris
Prostate Histotripsy: Feasibility and Safety
Several studies have been published confirming the feasibility [23, 29] and safety [29] of in vivo histotripsy prostate fractionation. Canine models are used since no other readily available animal model has a prostate sufficiently similar to the human. In these studies, anesthetized canine subjects were positioned supine. A 750-kHz, 18-element therapeutic ultrasound transducer was submerged in a degassed water bolus coupled to the shaved suprapubic region of the subject. A 10-MHz transrectal ultrasound imager provided high-resolution images of the prostate. Cavitation was applied in a grid configuration to treat a volume of tissue within the prostate, encompassing glandular and urethral portions of the prostate. Cavitation was confirmed and localized by identification of a bubble cloud on transrectal ultrasound imaging during application of acoustic energy. Following treatment, hypoechoic appearance of the targeted volume on ultrasound confirmed tissue homogenization. In a study of 18 canine subjects [29], prostates harvested at various time points (0, 7, 28, and 56 days) after histotripsy revealed no clinically significant collateral injury. Furthermore, clinical and laboratory assessment following treatment was notable for minimal gross hematuria without clots and only transient mild elevation of WBC, AST, and CK. Pain scores calculated from a validated canine pain scale [31] were consistent with mild posttreatment discomfort which resolved with urinary catheter removal 1–3 days after treatment. Histological assessment revealed evolution of patterns of tissue necrosis (coagulative, liquefactive, and hemorrhagic) and tissue homogenization consistent with patterns of normal wound healing. A mixed inflammatory response gave way to granulation tissue formation with angiogenesis and fibroblastic proliferation. The adjacent epithelium underwent varying forms of metaplasia (mostly squamous metaplasia) and re-epithelialization of the urinary channel (Fig. 24.9). With other treatment patterns, histological sections demonstrated a smooth square-shaped cavity corresponding to the volume targeted for treatment (Fig. 24.5).
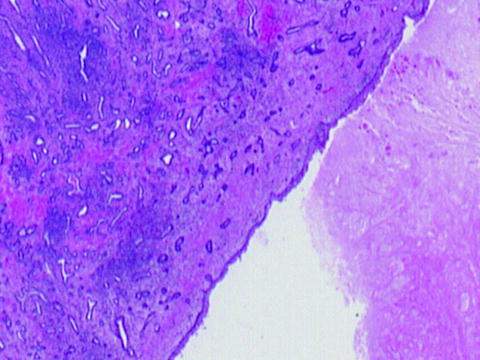

Fig. 24.9
Re-urothelialization of the treatment cavity is apparent 21 days after histotripsy treatment, suggesting healing without signs of fibrosis or contracture
Hemostasis
In the initial canine studies, it was intriguing that only mild gross hematuria was observed without clots or change in hematocrit despite creation of a TURP-like defect within in vivo prostates. This observation prompted a study in 9 subjects who were anticoagulated with warfarin (INR 1.2–11.3) prior to aggressive histotripsy treatment of the left hemi-prostate [32]. Similarly, only mild hematuria without clots was observed clinically, and serial assessment of hemoglobin did not demonstrate significant change. This suggests that the process of histotripsy homogenization may also have hemostatic properties as well. Perhaps the mechanical process that homogenizes tissue may also activate the coagulation cascade directly or induce signaling mechanisms via fractionation of structural and cellular components. Elucidation of the underlying phenomenon is necessary and will provide insight for further tailoring and optimization of histotripsy prostate therapy.
Periprostatic Structures: Thresholds for Mechanical Damage
Although the physical process of cavitation can be precisely applied in the ideal setting, other factors such as patient motion or errors in targeting have the potential to compromise focal prostate treatment or lead to collateral injury. It has also been observed that different tissue types and anatomic structures exhibit varying thresholds for damage from histotripsy. Histotripsy treatment of in vitro porcine kidneys was performed by packing individual treatment points 2 mm apart to fill a 5 × 5 × 15-mm target volume. This resulted in a complete cavity filled with liquefied debris when the target volume was within the renal cortex. Histotripsy of a similar volume encompassing renal cortex, medulla, and collecting system produced a different tissue effect. The collecting system was seen to be intact and undamaged after histotripsy treatment, while cortical tissue was homogenized and medulla sustained hemorrhage with small pockets of tissue damage [33]. A similar tissue-specific threshold for histotripsy damage has also been seen when targeting prostate tissue in an in vivo canine model. Periurethral dense stromal tissue required a greater number of pulses to produce tissue fractionation than did more peripheral, predominantly glandular tissue [30].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree