Hodgkin Lymphoma
Todd M. Blodgett, MD
Alex Ryan, MD
Barry McCook, MD
Key Facts
Terminology
Hodgkin lymphoma (HL); Hodgkin disease (HD)
Imaging Findings
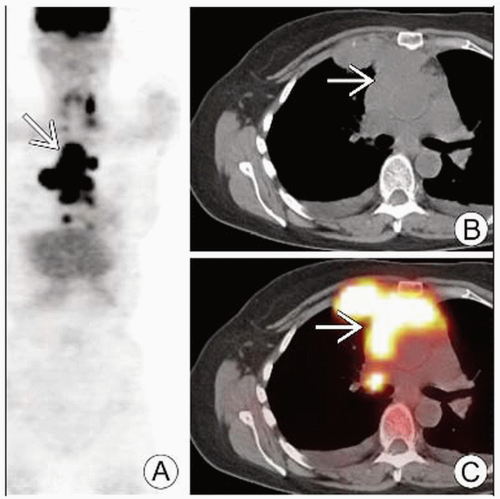
FDG PET/CT: Enlarged, hypermetabolic lymph nodes
PET/CT or PET + CT (side-by-side) is superior to contrast CT or PET alone for staging HD
Ga-67 inferior to FDG PET for initial staging
CECT can help evaluate cortical bone involvement but has low sensitivity in detecting bone marrow disease
Pooled true positive value of PET for HL 90% with upstaging rate ranging from 8-17% and downstaging rate from 2-23%
PET has prognostic value after 1-3 cycles of chemotherapy
After 2-3 cycles of chemo, PET-positive group had 39% 5 year survival vs. PET-negative group at 92%
For tumor response to therapy, low-dose CT PET/CT may be enough
PET has significant influence on staging of HL, upstaging 15-25% of patients with shift to more advanced treatment in 10% of patients
SUV reduction of 60% is used as cutoff to separate treatment responders from nonresponders
Top Differential Diagnoses
Granulomatous Process
Infections
Other Malignancy
Normal Lymphoid Tissue
Reactive Lymph Nodes
TERMINOLOGY
Abbreviations and Synonyms
Hodgkin lymphoma (HL)
Hodgkin disease (HD)
Lymph nodes (LN)
Definitions
Malignant neoplasm arising from lymphocytes
Rare variety is derived from histiocytes
IMAGING FINDINGS
General Features
Best diagnostic clue
FDG PET/CT
Enlarged FDG-avid lymph nodes/conglomerate mass
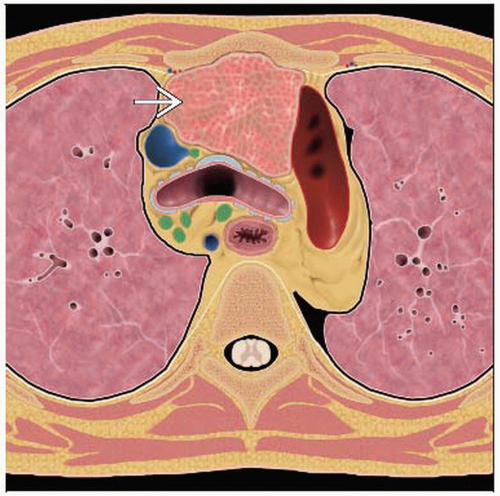
In usual location: Anterior mediastinum with other nodal groups
CT
Mediastinal lymphadenopathy presenting as mediastinal mass
± Hepatomegaly, splenomegaly, lung nodules/infiltrates, pleural effusions
Location
Uncommon spread to extralymphatic locations
CNS, spine
Usual spread is to contiguous lymph nodes
Then to viscera or bone marrow
30-40% of patients present with splenic involvement
5-14% of patients have bone marrow involvement
Bone involvement
Primary bone invasion does not affect staging; rare (1-4%) at presentation
Hematogenous spread indicates stage IV disease
Stage IV occurs in 5-20% of patients during disease course
6% of patients have chest wall involvement
Requires more aggressive therapy due to higher relapse rate
Thymic involvement considered “nodal”
Does not count as extranodal disease
Not associated with change in disease stage
Up to half of patients with thoracic HL may show enlarged thymus
Present after successful treatment as a result of rebound thymic hyperplasia
Occasionally develop thymic cysts
Rare locations
Peritoneal and omental involvement found only in non-Hodgkin lymphoma
Renal parenchyma is rarely involved, although perirenal space may be invaded
GI tract uncommon and usually due to nodal extension
Morphology
Rounded or bulky soft tissue mass due to nodal aggregation
Large masses may have areas of necrosis, hemorrhage, or cyst formation
Imaging Recommendations
Best imaging tool
PET/CT
Best for staging HD (sensitivity 94-98% and specificity 95-100%)
MR
To delineate soft tissue margins and evaluate spinal cord impingement
Protocol advice
Baseline FDG PET images should be obtained for initial staging
Prior to treatment
Patient should be kept warm and avoid activity prior to scan
Reduces physiologic uptake in muscle and fat
Low dose CT is acceptable for evaluating response to therapy
CT Findings
CT has replaced more complicated invasive diagnostic procedures
Method of choice for identification of disease invisible on clinical exam
Rarely performed anymore: Laparotomy/splenectomy, lymphangiography, and mediastinoscopy
Lymphadenopathy
Lymph node enlargement and aggregation
Appearance of multiple round or bulky soft tissue masses
Large masses may develop necrosis, hemorrhage, or cyst formation (10-20%)
Minimal contrast enhancement
Calcification rare before treatment but 20% prevalence post-radiotherapy
Rim calcification
Multiple discrete deposits (mulberry)
CT useful for treatment/radiation planning
Extralymphatic involvement
Mediastinal structures may show displacement, compression, or invasion
Cortical bone well evaluated with CT
Poor sensitivity for bone marrow disease
Invasion of gallbladder and pancreas usually from adjacent nodal disease
Absence of pancreatic capsule hinders diagnosis of invasion vs. contact
Thymic mass may be discrete or infiltrating
Therapy response
Tumor masses have low density of malignant cells
Reduction in volume of lesion is an insensitive predictor of response
Nuclear Medicine Findings
Initial diagnosis
Involved lymphoid tissue generally shows increased FDG uptake
No differentiation of subtypes by SUV has been demonstrated
Focal, super-physiologic uptake in nodal or extranodal tissue fairly specific indicator of disease
Diffuse uptake more difficult to interpret
Awareness of common FDG PET false positives is essential
Uptake may be seen in spleen and liver
Focal increased FDG activity generally indicative of malignant involvement
Staging
For organ staging, PET/CT seems to have no obvious advantage over FDG PET alone
Except in reducing false positives by better characterization of lesions using CT
Pooled true positive rate of FDG PET for HL: 90%
Upstaging rate: 8-25%
Shift to more advanced treatment: 10%
Downstaging: 2-23% (mean less than for upstaging)
FDG PET inclusion criteria are more accurate than CT inclusion criteria
Size is an insensitive indicator of malignancy
Enhancement characteristics are unreliable for inclusion
Combined PET/CT is superior for lesion delineation in radiotherapy planning
Bone marrow involvement
Diffuse marrow involvement may be intense
May also be indistinguishable from background
May be misinterpreted as negative for disease with diffuse marrow activity
Increased uptake can be iatrogenic
G-CSF, erythropoietin
Beta-thalassemia also increases uptake
Bone marrow biopsy (BMB) and PET/CT are complementary
Similar sensitivity/specificity but discordant findings
BMB more sensitive for detection of diffuse disease
PET/CT more likely to detect patchy disease
Spleen and liver
Full dose diagnostic CT necessary for adequate evaluation of liver and spleen
Splenic involvement may appear as diffusely increased uptake
Also seen in “reactive” spleen
Liver involvement may appear as diffuse uptake or patchy uptake in portal areas
Less commonly as large focal lesions
Response to therapy
SUV reduction of 60% is used as cutoff to separate treatment responders from nonresponders
PET has prognostic value after chemotherapy: 5 year survival after 2-3 cycles of chemo
92% for PET-negative group
39% for PET-positive group
2 year progression-free survival after 2 cycles of ABVD-like chemo
94% for PET-negative patients
0-6% for early PET-positive patients
Study showed no evidence that patients benefit from treatment alteration based on early PET
Patients with PET-negative residual mass after chemo who received radiotherapy to original bulky site had 2.5% relapse rate within 18 months vs. 14% relapse in non-radiotherapy arm
In contrast, the International Prognostic Index (IPS) poorly predicts improved survival
DIFFERENTIAL DIAGNOSIS
Granulomatous Process
Active disease positive on FDG PET
Infectious and non-infectious etiologies
Will usually resolve over time
More likely bilateral hilar and paratracheal distribution
Infections
Pyogenic, fungal, parasitic, HIV-related, viral (e.g., varicella, zoster, HCV, CMV)
Usually positive on FDG PET
Other Malignancy
Variable enhancement and FDG uptake
History is crucial
Normal Lymphoid Tissue
Physiologic uptake common in Waldeyer ring, thymic tissue, cervical nodes
Asymmetric uptake can occur normally and may be mistaken for malignancy
Reactive Lymph Nodes
Usually much smaller than typical aggressive Hodgkin
PATHOLOGY
General Features
Genetics
1% of patients with HD have family history of disease
Sibling of affected individual has 3-7x increased risk
Higher in monozygotic twins
HLA-DP alleles more common in HD
Etiology
Unknown
Infection may be involved in pathogenesis, particularly Epstein-Barr virus (EBV)
Tumor cells are EBV-positive in ˜ 50% of HD cases
Positivity higher in MCHD (60-70%) than in NSHD (15-30%)
˜ 100% of HIV-related HD are EBV-positive (though HD is not an AIDS-defining condition)
Epidemiology
8,000 new cases and 1,000 deaths occur in the USA annually
Incidence: 3-4/100,000 per year
Gross Pathologic & Surgical Features
Cut surface white-gray and uniform
Affected LN
Usually enlarged
Shape is preserved
Capsule is not invaded
Surface may be nodular in nodular sclerosis subtype
Microscopic Features
Prominent lymphocytic infiltrate and Reed-Sternberg cells
Reed-Sternberg cells: Large, binucleate, with characteristic CD15+, CD30+ immunophenotype
Core biopsy preferred over fine needle aspiration
Malignant cells in HL make up only a very small, scattered proportion of the tumor volume
Subtyping requires core biopsy
Tumors pleomorphic
Bone marrow disease is often patchy and focal resulting in low sensitivity of bone marrow biopsy
If positive on PET, directed biopsy of that area increases true positive yield
Staging, Grading, or Classification Criteria
Staging criteria
Physical examination
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree