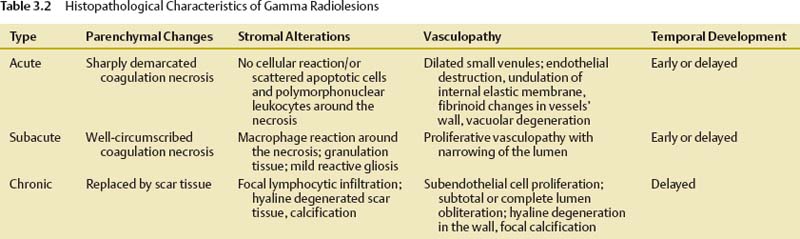
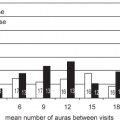
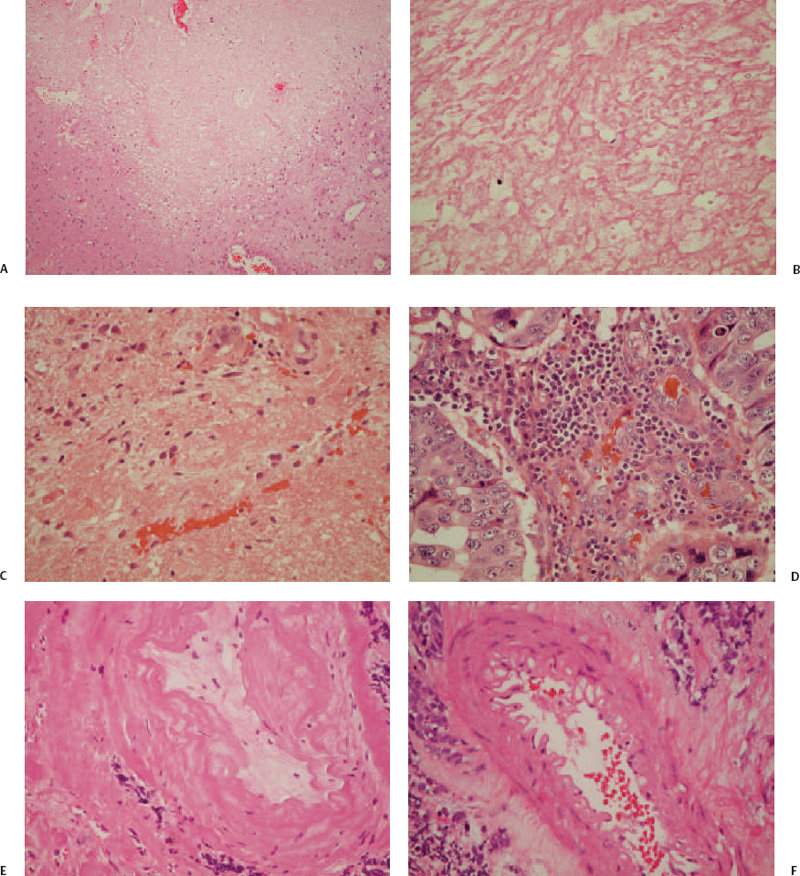
3 Tumor radiosurgery has become an effective and widely used procedure during the past 4 decades.1–10 The first Gamma Knife radiosurgery patient had a craniopharyngioma. The radiobiological effect of radiosurgery is not well understood.11,12 Although more than 200,000 patients with benign or malignant intracranial neoplasms have undergone Gamma Knife radiosurgery worldwide, the histopathologic response leading to tumor control or continued growth has received limited attention.13–17 The purpose of this study was to define the types of histopathological changes as determined by analysis of tumor specimens from patients who required resection for either tumor progression or delayed intratumoral hemorrhage. From a series of 5,000 brain tumors in the University of Pittsburgh Gamma Knife program, surgical pathology material was reviewed from 19 patients who underwent 24 tumor resections at 2 to 82 months (median 13 months) after radiosurgery. All patients had radiological and clinical progression that warranted removal of the lesion via craniotomy. Tumors included metastases (n = 15), anaplastic astrocytoma,2 atypical meningioma,2 vestibular schwannoma,2 and one meningioma, jugular foramen schwannoma, and hemangioblastoma. Radiosurgery was performed using the Gamma Knife (Elekta Instruments AB, Stockholm, Sweden) models U, B, or C. Dose planning was based on magnetic resonance (MR) and computed tomography (CT) imaging. Tumor volumes varied between 0.266 and 25.600 mL (median 4.7 mL). Tumors received 12 to 20 Gy as the margin dose (median 16 Gy) at the 30 to 60% isodose line (median 50%), with a 24 to 40 Gy maximal dose (median 32 Gy). Histopathological investigations were performed on all available surgical pathology materials. Resected specimens were fixed in 10% neutral buffered formaldehyde and embedded in paraffin. Hematoxylin-eosin, Masson’s trichrome, and Luxol-fast blue stains were used. Immunohistochemical reactions included glial fibrillary acidic protein (GFAP), vimentin, S100, neurofilament, synaptophysin, EMA, pankeratin, CK7, CK20, CAM5.2, CD3, CD20, CD31, and CD68 (PGM1) antigens to characterize the reactive cell population around/or infiltrating neoplastic tissues. Ki67 and p53 reactions were used to assess the proliferative activity of tumor cells. Biotin-streptavidin-peroxidase complex methods were performed according to standard protocols on 5 μm paraffin sections. Histopathological changes attributable to radiosurgery were observed within the tumor parenchyma, adjacent connective tissue stroma, and vessels (Table 3.1). These changes were either degenerative or proliferative. The main histopathological characteristics are summarized in Table 3.2. Degenerative alterations occurred mostly in the parenchyma of different tumors, whereas proliferative processes were realized first of all in the connective tissue stroma and vessels of neoplasms. The basic histopathological alteration evoked by the ionizing energy of high-dose radiation was recognized as a well-circumscribed lesion with sharp demarcation toward surrounding tissues according to the steep radiation fall-off (Fig. 3.1A). Regarding the histological and cellular composition of these gamma radiolesions, three main types were realized following radiosurgery (Table 3.2). In the acute-type reaction, coagulation necrosis constituted the center with a network of acidophilic fibrinoid material and amorphous homogeneous tissue or cellular debris (Fig. 3.1B). No cellular reaction or scanty basophilic hyperchromatic apoptotic cells characterized by nuclear fragmentation and pyknosis intermingled with scattered polymorphonuclear leukocytes and some dilated postcapillary venules surrounded this necrotic core (Fig. 3.1C). There was no prominent macrophage or lymphocytic infiltration, reactive gliosis, or scar tissue formation. The necrosis in this targeted volume was usually circumscribed, homogeneous, and sharply demarcated from surrounding remaining tumor tissue (Fig. 3.1A) as compared with other necrotic tumor areas outside the focused irradiation, which had an irregular multifocal appearance and entrapped tumor islands (Fig. 3.1D). A similar histological picture of the acute-type lesion could be observed at either an early or a delayed time interval after radiosurgery. These parenchymal changes were accompanied by alterations of stromal vessels around the necrotic core characterized by endothelial destruction, fibrinoid necrosis, undulation of the internal elastic membrane, and vacuolation and accumulation of eosinophilic material (transudation) in the vessel wall (Fig. 3.1E,F). Fig. 3.1 (A-D) Histological characteristics of acute-type gamma radiolesions from a metastatic non–small cell carcinoma 4 months after Gamma Knife radiosurgery. (A) A sharply demarcated coagulation necrosis in the tumor parenchyma (hematoxylin-eosin [H. & E.], × 100). (B) Fibrinoid mesh in the center of the radiolesion (H. & E., × 300). (C) Scattered pyknotic cells at the periphery of the lesion (H. & E., × 300). (D) Irregular tumor necrosis outside the irradiated target volume (H. & E., × 300). (E) Fibrinoid necrosis in the vessel’s wall (H. & E., × 300). (F) Endothelial destruction, undulation of internal elastic membrane, and vacuolar degeneration of the vessel’s wall (H. & E., × 300). (E and F are from a metastatic small cell lung carcinoma 3 months following Gamma Knife radiosurgery.) The second group of gamma radiolesions had the characteristics of subacute-type pathological reactions that were observed several months to years after radiosurgery. The main histological feature of these lesions was an inflammatory tissue response. The central coagulation necrotic core was surrounded by a macrophage rim (Fig. 3.2A). These macrophages revealed phagocytotic activity and mainly CD68 (PGM1) but sometimes CD31 immunohistochemical reactivity as well (Fig. 3.2B). A granulation tissue zone was situated outside the macrophage layer containing abundant small vessels, capillaries, arterioles, and venules accompanied by inflammatory cells, fibrocytes, and fibroblasts expressing vimentin positivity (Fig. 3.2C,D). Postirradiation vasculopathy was also observed at the periphery of the central necrotic region (Fig. 3.2E.F). A reactive gliotic scar mantle formed by astrocytic elements and abundant glial filaments coated and constituted the outer border of the radiolesion as it was demonstrated by GFAP immune reaction (Fig. 3.2G,H).
How Brain Tumors Respond to Stereotactic Radiosurgery
György T. Szeifert, Dave S. Atteberry, Douglas Kondziolka, and L. Dade Lunsford
 Patients and Methods
Patients and Methods
 Results
Results
Acute Changes
Subacute Changes
How Brain Tumors Respond to Stereotactic Radiosurgery