Introduction
Carotid occlusion is one of the most important diagnoses that is made in the vascular laboratory or imaging department. The distinction between carotid occlusion and near-total occlusion or high-grade stenosis is critical as treatment options remain available for severe carotid stenosis. In this chapter, we discuss the significant findings associated with carotid occlusion and provide insights to distinguish between high-grade stenosis and occlusion. We also review rare and uncommon carotid artery pathologies that you need to know. Although these pathologies are not commonly encountered, the correct identification of these carotid conditions is critical to appropriate patient care.
Carotid Occlusion
Although not often seen, distinguishing a complete carotid artery occlusion from a near-total carotid artery occlusion is one of the most important decisions made during a carotid evaluation. The finding of carotid occlusion carries significant clinical implications because this diagnosis precludes therapeutic options, including endarterectomy and stent placement. The diagnosis requires careful evaluation of the occluded segment as well as the inflow and outflow vessels. Review of the technical parameters must be performed to avoid pitfalls and misdiagnosis. Failure to detect blood flow in the carotid artery may be related to low-flow state, poor Doppler sensitivity, limited visualization of the carotid artery, near-total occlusion, or carotid occlusion. In this section, we review the findings and pitfalls associated with confirming the presence of a carotid artery occlusion.
Atherosclerosis is by far the most common cause of carotid artery occlusion, but fibromuscular dysplasia and arterial dissection (discussed later) are additional causes. Patients may be acutely symptomatic or asymptomatic during presentation. Most occlusions in the carotid system occur in the internal carotid artery (ICA), but occlusion also may occur in the common carotid artery (CCA) or external carotid artery (ECA). The incidence of CCA occlusion occurs approximately one-tenth as often as ICA occlusion but is sufficiently common to be seen in a typical community-based vascular practice. CCA occlusion is frequently accompanied by stroke or other neurologic events but also may be encountered in the absence of neurologic symptoms. The ICA may remain patent in spite of CCA occlusion ( Fig. 8.1 ) because collateral supply to the ICA develops through the ipsilateral ECA branches. Flow reverses in the collateralized ECA branches and remains cephalad in the ICA.

Optimization of transducer frequency and position is important to assess the carotid vessels. Careful optimization of the color, power, and pulsed Doppler modes is essential for correct diagnosis. The color gain, color velocity scale or pulse repetition frequency (PRF), and color wall filter must be optimized for slow flow in order to adequately assess for patency of the artery. Similarly, the pulsed Doppler gain, PRF, and wall filter must be adjusted to detect blood flow in the vessel during spectral analysis. Thorough interrogation of the vessel lumen with an ample sample volume is required.
Carotid occlusion is recognized on Doppler studies as the absence of blood flow in the carotid artery with color, power, and pulsed Doppler. No flow is detected on the spectral (pulsed Doppler) display at low PRF settings. Remember, pulsed Doppler is more sensitive than color Doppler for the detection of slow- or low-velocity flow. Do not mistake the highly damped arterial flow signals for venous flow. Be sure to check the flow direction . Finally, look at the occluded vessel from several transducer approaches, including the transverse plane, before concluding that flow is absent. See Table 8.1 .
| Absence of flow in occluded segment with color, power, and pulsed Doppler |
| Echogenic material filling the ICA lumen |
| Prominent collateral vessels |
| Rocking flow pattern in distal CCA |
| Low-velocity, high-resistance flow in distal CCA |
| Low-resistance flow in ECA (“internalization of the ECA”) |
| Increased flow velocity in contralateral CCA and ICA (“compensatory flow”) |
| Small vessel size in chronic occlusion |
Look for clues during the exam to help determine the duration of occlusion. Fresh thrombus is hypoechoic and may require increased gray-scale settings to visualize. Echogenic material may be seen filling the lumen of the artery in subacute or chronic occlusion. The vessel may be small in size in a chronic occlusion. Prominent collateral vessels may be seen from ECA branches with chronic occlusion.
There are important indirect signs of ICA occlusion. A pulsatile or rocking flow pattern commonly called the “thump-in-the-stump” sign on the Doppler spectrum is seen in the distal CCA proximal to the site of occlusion. This is related to antegrade blood flow striking the obstructed segment in systole and reversing in diastole. This produces a back-and-forth motion in the proximal segment. The Doppler waveforms obtained in the CCA show a low-velocity, high-resistance waveform with decreased, absent, or reversed flow in diastole ( Fig. 8.2 ). Increasing resistance is usually noted in the distal CCA, as one samples closer to the occlusion, because of outlet obstruction. There may be increased low-resistance flow in the ECA as collaterals attempt to increase blood flow on the ipsilateral side. This is called “internalization of the ECA” and reflects collateral flow to the brain. Increased flow velocities may be seen in the contralateral ICA with ipsilateral ICA stenosis or occlusion. This is referred to as “compensatory flow of the contralateral ICA” and is another mechanism to increase blood flow to the brain.

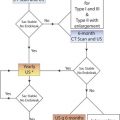
False-positive diagnoses may occur when the artery is obscured by acoustic shadowing from calcified plaque, when image quality is poor, when Doppler signals are weak, and especially when the vessel is nearly occluded and only a “trickle” of flow is present in near-total occlusion of the artery. A near occlusion of the ICA is associated with the angiographic “string sign” ( Fig. 8.3 ). It is important to realize that the apparent very small caliber of the distal ICA called the “string sign” on contrast arteriography is an artifact. The string sign results from puddling of the slow-moving contrast agent in the dependent (posterior) portion of the arterial lumen with the patient supine. The distal lumen may in fact be larger, and the stenosis located only at the ICA origin. See Table 8.2 .

| Carotid artery is obscured by calcified plaque |
| Poor image quality |
| Slow “trickle flow” from near-total occlusion |
| Low color gain or high pulse repetition frequency and wall filter |
Distinguishing carotid occlusion from high-grade carotid stenosis can be a challenging task for the sonographer or sonologist. Low flow in a near-total occlusion may be difficult to detect with preset Doppler parameters and requires optimization of color, power, and pulsed Doppler settings. The use of power Doppler imaging is recommended because of its sensitivity to low flow rates. Studies using color or power Doppler report close to 100% sensitivity and specificity in the diagnosis of near occlusion of the ICA. To attain this level of accuracy, however, several technical details must be followed. First, adjust the instrument to detect minimum flow velocity. The PRF should be as low as possible, and the low-frequency filter should be minimized so that low-frequency signals are not excluded. Second, obtain the best possible view of the occluded vessel and scrutinize the lumen for any hint of blood flow. Remember that the view chosen should optimize the Doppler angle of the color flow image. Toggling between color and power Doppler is helpful especially when motion artifacts are a problem. Third, interrogate the visualized segments of the carotid artery with spectral Doppler. The signals obtained may be very weak, so high Doppler gain and low velocity settings are needed.
- •
Failure to detect blood flow in the carotid artery may be related to low-flow state, poor Doppler sensitivity, limited visualization of the carotid artery, near-total occlusion, or carotid occlusion.
- •
Pulsed Doppler is more sensitive than color Doppler for the detection of slow- or low-velocity flow.
- •
Low flow in a near-total occlusion may be difficult to detect with preset Doppler parameters and requires optimization of color, power, and pulsed Doppler settings.
- •
To distinguish carotid occlusion from high-grade stenosis with low flow, be sure to adjust the instrument to detect low flow, obtain the best possible view of the lesion, use power Doppler, and check with pulsed Doppler interrogation.
Carotid Artery Dissection
Arterial dissection refers to the entry of blood into the wall of the artery, separating the layers of the wall, and creating a false lumen through which blood flows. For blood to enter the wall and cause dissection, there must be a rent in the intima, which may be caused by violent trauma, iatrogenic trauma, or an underlying weakness of the muscular layer that allows the intima to tear. The location where the wall layers separate varies. In some cases, only the intima is dissected from the wall, whereas in other cases, portions of the media or the media and adventitia may delaminate. Thus the thickness of the membrane separating the true and false lumens varies. Dissection through the adventitial layer may allow pseudoaneurysm formation adjacent to the artery.
Arterial dissection produces a false lumen that may be blind-ended or may reconnect with the true lumen at a site distal to the point of dissection. A blind-ended false lumen can thrombose (occlude) and bulge into the true lumen, causing stenosis or occlusion. Blood continues to flow in the false lumen if its distal end reconnects with the true lumen. Following carotid dissection, embolization or reduced flow may cause thrombosis of intracranial vessels and brain damage. Less commonly, pseudoaneurysm occurs when dissection extends to the serosal or periadventitial layer of the carotid artery.
CCA dissection usually originates in the aortic arch and extends only to the carotid bifurcation, but dissection can extend into the ICA. Three to seven percent of aortic arch dissections are complicated by stroke or transient cerebral ischemia. Common carotid extension most often occurs with ascending arch dissection (Stanford type A), which usually is related to atherosclerotic disease but also may be caused by elastic tissue degeneration, as seen with Marfan or Ehlers-Danlos syndromes. Stanford type B dissection, occurring distal to the aortic arch, usually does not affect the carotid arteries. Considering that neurologic symptoms are uncommon when carotid dissection extends from the aortic arch, carotid dissection is occasionally an incidental finding at carotid sonography.
Carotid dissection may also originate within the ICA, and may occur either spontaneously or following trauma. Some “spontaneous” dissections may not really be spontaneous at all but may actually result from minor trauma, such as strenuous exercise or rapid neck motion. For example, “spontaneous” dissections have been associated with sneezing and coughing. In some cases, the precipitating trauma may be unrecognized by the patient. Arterial pathology may also lead to atraumatic ICA dissection, including fibromuscular dysplasia (FMD), Marfan syndrome, cystic medial necrosis, and Ehlers-Danlos syndrome. Unlike CCA dissection, the false lumen of ICA dissection is almost always occluded by thrombus. These lesions typically occur in the mid to distal portions of the ICA.
Seventy percent of spontaneous ICA dissections or those following minimal trauma occur in patients 35 to 50 years of age, with an equal incidence in men and women. Systemic hypertension is present in one of three cases and is considered a predisposing factor. Presentation includes headache, neck and facial pain, hemispheric ischemic symptoms, and cranial nerve palsy. ICA dissections account for approximately 20% of strokes in patients less than 45 years of age. Spontaneous restoration of ICA flow occurs in many cases of high-grade stenosis or occlusion through retraction of the thrombus in the false lumen, relieving compression of the true lumen. Therapies used for carotid dissection are usually antithrombotic and antihypertensive medications.
Carotid dissection resulting from violent trauma is related to direct injury to the ICA, usually from stretching of the artery across cervical spine structures or from direct arterial compression by cervical spine elements or the mandible. Trauma causes a rent in the intima and an injury that weakens the underlying wall structure, permitting delamination. Serious neurologic consequences are more common with traumatic carotid dissection than with atraumatic dissection. Mortality was 7% for patients without ischemic stroke and 32% for patients with stroke.
The ultrasound findings associated with common carotid dissection may be dramatic when the intima is separated from the rest of the wall and flutters in the flow stream with each cardiac cycle ( Fig. 8.4 ). Severe flow disturbances are caused by the flapping intima ( Fig. 8.5 ). However, if the tissue between the true and false lumens is thick, the intervening membrane is stiffer and dissection is indicated simply by duplication of the carotid lumen on color Doppler.


The classic sonographic presentation of ICA dissection is a smooth, tapering stenosis ( Fig. 8.6 ) occurring in a patient who is younger than the typical patient with atherosclerotic stenosis (i.e., <50 years of age). Regardless of age, however, consider dissection in any patient with a smooth, tapering ICA stenosis without visible atherosclerotic plaque . In some cases of ICA dissection, the sonographic findings may be subtle and easily overlooked. The ICA lumen may be normal in the area just above the bifurcation if the dissection begins at the skull base and does not extend down to the point of sonographic visualization. In such instances, the only detectable abnormality is increased flow resistance seen in the Doppler waveforms and possibly reduced flow velocity overall due to distal ICA obstruction.

The demonstration of an intimal flap allows for definitive diagnosis of carotid dissection. On occasion, a thin intimal flap may not be appreciated during color Doppler examination because of color-blooming artifact. That is, the color will obscure (“write over”) the thin flap, and only the color flow disturbance is seen. The flap is better seen when the examiner turns off the color display and examines the vessel with gray-scale imaging. When a patient presents with ICA occlusion with no apparent cause, consider the diagnosis of carotid dissection. Again, the absence of atherosclerotic plaque or youthful presentation should suggest the diagnosis of dissection.
When carotid dissection is recognized, several pieces of information should be obtained. First, the extent of dissection should be ascertained, which in turn may indicate whether dissection originated in the aortic arch or the ICA. Second, the presence, direction, and characteristics of flow in the true and false lumen are documented. Third, the patency of the ECA and ICA is determined, and Doppler waveforms from both vessels are analyzed to assess the status of the ICA circulation and the presence of ECA collateralization. Finally, if dissection causes stenosis, the degree of narrowing should be evaluated both visually (color flow) and with Doppler velocity measurements. Further evaluation with conventional arteriography, magnetic resonance angiography (MRA), or computed tomographic angiography (CTA) is usually performed to appreciate the full extent of dissection.
- •
Common carotid artery dissection usually originates in the aortic arch and can extend to the carotid bifurcation, and into the ICA.
- •
Carotid dissection may also originate within the ICA and may occur spontaneously or following trauma. These lesions tend to occur in the mid and distal segments of the ICA.
- •
Consider dissection in any patient with a smooth, tapering ICA stenosis without visible atherosclerotic plaque .
- •
The demonstration of an intimal flap allows for definitive diagnosis of carotid dissection.
Carotid Pseudoaneurysm
A pseudoaneurysm, or false aneurysm, is a vascular mass that results from a hole in the arterial wall with circulating blood flow that is confined by soft tissue and hematoma. A true aneurysm is one in which the artery walls are intact but stretched. Carotid pseudoaneurysms most often result from violent trauma (i.e., knife or gunshot wound) but also occur iatrogenically from attempted percutaneous jugular vein catheterization or during therapeutic/diagnostic arteriography. Additional causes are carotid dissection or pathologies that weaken the arterial wall, such as vasculitis, fibrous dysplasia, Marfan syndrome, and Ehlers-Danlos syndrome.
Iatrogenic and posttraumatic pseudoaneurysms are usually accompanied by considerable ecchymosis, hematoma, or other trauma-associated findings. Pseudoaneurysms caused by nonpenetrating trauma, arterial diseases, or catheterization may present only with a palpable, and usually pulsatile mass, neck pain, or cranial nerve palsy. Neurologic symptoms of any kind, including cerebral ischemia/stroke, are reported in 40% of cases, and these may be accompanied by imaging evidence of cerebral infarction. Perhaps the most dramatic consequence of carotid pseudoaneurysm is rupture and life-threatening soft tissue hemorrhage, but this occurs only rarely. Until recently, therapy for carotid pseudoaneurysm has been surgery, but clinically stable lesions may now be treated nonsurgically with covered wall stents.
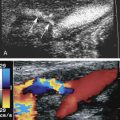
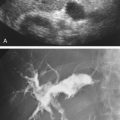
Sonographically, carotid pseudoaneurysms are spherical perivascular lesions into which blood is seen to circulate from the carotid artery ( Fig. 8.7 ). The size of the lesion is variable, as is the relative proportion of thrombus and circulating blood within the pseudoaneurysm. Some pseudoaneurysms may be largely thrombosed, with only a small amount of blood flow. Other lesions may show large areas of swirling blood flow with little thrombus. In all cases, however, a to-and-fro flow pattern should be seen in the neck of the pseudoaneurysm on spectral Doppler examination, similar to pseudoaneurysms found in the groin. The distance of the pseudoaneurysm from the carotid artery also is variable, and the length of the “neck” connecting the two varies from one case to another. The diameter of the neck also varies.