Author(s)
Year
Number of cases
Sex ratio (M:F)
Mean age (years)
Gupta et al.
5
3:2
13.4
Khaldi et al.
117
Slight male predominance
7.2
Bükte et al.
18
2:1
20.3 (7–50 years)
Ciurea et al.
76
45:31
Children: 8.7
Adults: 29.3
Tlili-Graiess et al.
25
0.78
14 (30 months–56 years)
Ali et al.
21
1:1.3
7.42 ± 3.2
Duishanbai et al.
30
21:9
11
Basraoui et al.
9
1:1
7.5
Mohindra et al.
9
7:2
7 (4–44 years)
In humans, intracranial hydatidosis can be associated with involvement of other organs such as liver or lung, or it may be an isolated infestation of the brain. Only embryos which succeed in passing through the filtering barrier systems in the liver and lung reach the brain by the systemic circulation. Once the hexacanth embryo has reached the brain, it will form a hydatid cyst because of the ideal growth conditions (Turgut 2001). Although hydatid disease may be located anywhere in the brain, it most frequently involves the cerebral hemispheres (90–95 % of the cases) (Abbassioun and Amirjamshidi 2001). Other reported sites of intracranial hydatid cysts include subarachnoid space, ventricles, pons, cerebellum, aqueduct of Sylvius, extradural space, and diploic space of skull bones (Gokalp and Erdogan 1988; Cemil et al. 2009; Furtado et al. 2009).
Brain hydatid cysts are most often localized supratentorially in the distribution of the terminal branches of the middle cerebral artery, usually temporo-parieto-occipitally (Behari et al. 1997; Tuzun and Hekimoglu 1998). Left hemispheral predominance has been noted in several series in the literature and is explained by the emergence of the left common carotid artery directly from the aortic arch (Ciurea et al. 2006). Three forms of intracranial hydatid disease occur: intracerebral, intracranial extradural, and a combined form (Samiy and Zadey 1965; Ba’assiri and Haddad 1984; Canbolat et al. 1994; Cemil et al. 2009). Intracranial epidural hydatid disease is extremely rare because the physiologic flow of blood to the brain is mainly through the internal carotid system, so the likelihood of the larvae traveling through the external carotid system is very rare (Samiy and Zadey 1965; Ba’assiri and Haddad 1984; Canbolat et al. 1994).
Multiple hydatid cysts of the brain are very rare. Primary multiple cysts of the brain resulting from arterial embolism and without any radiological or clinical evidence of hydatid disease elsewhere in the body are extremely rare (Iplikcioğlu et al. 1989; Gupta et al. 1991; Mancuso et al. 1997; Ozkan et al. 2001; Karadağ et al. 2004). In a review of the literature related to primary multiple intracranial cerebral hydatid cysts, we were able to find only 19 cases (Table 6.2). Secondary multiple hydatid cysts of the brain can result from spontaneous, traumatic, or surgical rupture of a primary solitary cerebral cyst or as a consequence of a cyst rupture elsewhere and embolization of hydatids to the brain (Nurchi et al. 1992; Martin Oterino et al. 1996; Mancuso et al. 1997; Anvari et al. 2009). Multiple hydatid cysts resulting from the rupture of a primary cyst are acephaloceles; they are infertile and have no brood capsule and scolices (Lunardi et al. 1991; Ciurea et al. 2006; Onal et al. 2006).
Table 6.2
Summary of the previously reported primary multiple cerebral hydatid cysts
Author(s), year(s) | Age/sex | Localization | Treatment | Outcome |
|---|---|---|---|---|
Sharma et al. (1982) | 9/F | 5 cysts in the right supratentorial region | Surgery | Good |
Todorov et al. (1988) | 45/M | 8 cysts in the frontal, temporal, parieto-occipital regions | Albendazole | Good |
Paşaoğlu et al. (1989) | 15/M | 3 cysts in the left frontoparietal and 1 cyst in the left occipital regions | Surgery | Good |
İplikcioğlu et al. (1989) | 7/F | 3 cysts in the right frontal, 2 cysts in the left occipital,1 cyst in the left frontal regions | Surgery | Good |
Cataltepe et al. (1991) | 8/M | 2 cysts in both parieto-occipital regions | Surgery | Good |
Gupta et al. (1991) | 18/M | Multiple cysts in both cerebral and left cerebellar hemispheres | Surgery | Died |
Nurchi et al. (1992) | 9/M | 30 cysts in the right parietal and occipital regions | Surgery | Good |
Bilge et al. (1993) | 37/M | 2 cysts in the left frontoparietal and occipital regions | Surgery | Good |
Martin Oterinoet al. 1996 | 69/F | Multiple cysts in both cerebellar and temporal regions | Albendazole | Died |
Mancuso et al. (1997) | 62/M | 1 cyst in the right frontal and 1 cyst in the left frontal regions | Surgery | Good |
Baysefer et al. (1998) | 20/M | 20 cysts in the left frontoparietal regions | Surgery | Good |
Popli et al. (1998) | 20, M | Multiple cysts in the left temporoparietal region | Surgery + albendazole | Good |
Ozkan et al. (2001) | 8, M | More than 25 cysts in the left temporo-parieto-occipital region | Surgery | Good |
Nowak et al. (2002) | 46, F | 2 cysts in the cerebral and cerebellar regions | Albendazole | Good |
Karadağ et al. (2004) | 45, F | 2 cysts in the right parietal region | Surgery | Good |
Yurt et al. (2007) | 19, F | 24 cysts in both cerebral hemispheres | Surgery + albendazole | Good |
Erkutlu et al. (2008) | 15, M | 2 cysts in the right parietal and occipital regions | Surgery | Good |
Cavusoglu et al. (2009) | 15, M | 19 hydatid cysts in the right parieto-occipital region | Surgery | Died |
Primary hydatid infestation caused by embryos which escaped hepatic and pulmonary barriers is generally single and fertile. On the other hand, secondary hydatidosis caused by scolices from ruptured fertile cysts is usually multiple and sterile. It is usually caused by myocardial cysts rupturing into the left ventricle. In a review of 112 children with cerebral hydatid cysts, Carcassone et al. (1973) found only four cases of multiple cysts in the brain (4 %). In the meta-analysis of Turgut (2001), there were 53 cases of multiple cysts (19 %), and the source of secondary multiple cysts was the left ventricle of the heart in two patients. For this reason, in such cases the heart must be carefully investigated as the source of the secondary multiple intracranial cysts. There is no consensus on the growth rate of the hydatid cyst of the brain and has been variably reported between 1.5 and 10 cm per year. Importantly, hydatid cyst has been shown to grow faster in the cerebrum than in the liver by a ratio of 3:1 revealing that brain like the lung tissue facilitates growth of the cyst as it is a compressible organ (Abbassioun and Amirjamshidi 2001; Andronikou et al. 2002). According to a review by Duishanbai et al. (2010), supratentorial hydatid cysts tend to be larger than the infratentorial counterparts when causing symptoms.
Diagnostic Approach
Quite independently of the hydatid cyst localization and the circumstances of its discovery, the diagnostic approach is always the same based on epidemiological, clinical, biological, imaging, and pathological arguments.
Epidemiological Data
Patients are usually from rural areas; they may have hydatid contact and live in canine environment. Children are in close contact with dogs. People are not practicing proper sanitary procedures and eating raw and uncooked or improperly cooked vegetables contaminated with Echinococcus ova.
Clinical Data
Intracranial hydatid cyst presents with a wide range of clinical manifestations which mainly depend on the location and size of a given hydatid cyst (Altinörs et al. 2000). Cysts develop insidiously, usually being asymptomatic initially, and present with protean clinical and imaging features (Ersahin et al. 1993). Headache and vomiting due to raised intracranial tension are the most common presenting features (Carrea et al. 1975; Ersahin et al. 1993). Other manifestations of cerebral hydatid cysts are seizures, hemiparesis, visual disturbances, and ataxia. Papilledema is usually present at the time of presentation in children with cerebral hydatid cysts. Evidence of pyramidal tract dysfunction, appropriate to the location of the cyst, is noted in almost 90 % of patients. Mental changes are noted in 25 % of children and seizure activity in approximately 20 %. Other symptoms such as hemiparesis, seizures, visual field alteration, and gait disorders may vary with the location of the cyst (Paşaoğlu et al. 1989; Ozek 1994; Yuceer et al. 1998). According to some authors, clinical presentation of cerebral hydatidosis is somewhat different in children and adults (Micheli et al. 1987; Ersahin et al. 1993). Signs of increased intracranial pressure with papilledema dominate in the younger age group, whereas focal findings such as hemiparesis, speech disorders, and hemianopsia, sometimes associated with epileptic seizures, are more prevalent in the older age group (Micheli et al. 1987; Ersahin et al. 1993). The disparity between the size of the cyst and clinical presentation is an important feature of cranial hydatid cysts (Andronikou et al. 2002). In addition, duration time of symptoms is found to be an independent predictor of the outcome (Duishanbai et al. 2010).
Biological Diagnosis
Routine work-up of hydatid disease comprises blood investigations and serological tests. Blood investigations are abnormal in only few patients who demonstrate raised eosinophilic count. The serological tests are of little practical value in confirming the diagnosis of cerebral echinococcal disease (Popli and Khudale 1998). In systemic hydatidosis, serological test (Casoni skin test) is generally positive. However, in cerebral and intracranial hydatidosis, these serological tests are almost always negative (Baden and Elliot 2003).
Nonspecific Biology
The hemogram inconstantly shows hypereosinophilia.
Specific Biological Diagnosis of Hydatidosis
Direct Evidence of Parasitic Elements
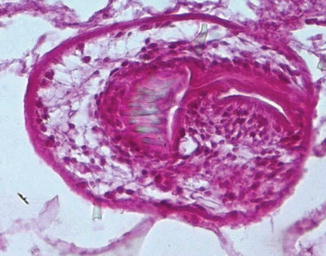
Direct evidence of parasitic elements can be obtained from surgical samples. The parasitic elements to be sought are the cyst membranes, protoscolices, and hooklets. Although radiological investigations can be very helpful in identifying hydatid cysts preoperatively, their diagnosis is mainly based on histopathological examination (Figs. 6.1 and 6.2).
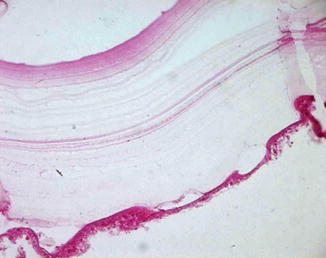

Fig. 6.1
Microscopic picture showing the laminated membrane and the germinal layer of the cyst (Haematoxylin and Eosin, magnification × 400)