Image-Guided Brain Tumor Resection
Surgical treatment of primary brain tumors remains a considerable challenge for the neurosurgeon. Nonetheless, surgery is the mainstay of glioma therapy because it provides the means of obtaining absolute tissue diagnosis, mapping tumor margins, mechanical cytoreduction, and guidance of adjuvant therapy such as brachytherapy, gene therapy, or radiosurgery. Recent developments in computation technology have fundamentally enhanced the role of medical imaging, from diagnosis to computer-aided surgery. Today, computer-assisted methods provide real-time information for dynamic navigation, analysis, and inspection of three-dimensional (3-D) image structures for preoperative surgery, virtual surgery, and intraoperative localization.
Kelly’s pioneering work to establish volumetric stereotactic resection techniques has provided a basis for assessing the benefits of surgical resection of deeply seated parenchymal tumors.1 These techniques allow for proper diagnosis and definitive resection of benign and circumscribed gliomas as well as for radical cytoreduction of the bulk of high-grade tumors. Advances in image-guided technology greatly enhance a surgeon’s ability to create a plan prior to surgery, to follow it during surgery, and to modify the surgical approach based on intraoperative information.
Although extent of resection of gliomas has not been analyzed as an influence on outcome in a prospective randomized trial, recent studies suggest improved length of survival after aggressive surgical resection.2–7 Berger et al8 have demonstrated that the degree of resection and amount of residual tumor are significantly related to the incidence of recurrence and to the tumor grade at recurrence. In the past, the task of correlating preoperative and intraoperative imaging studies was left to surgeons and depended on their knowledge of human anatomy. Stereotaxy enabled neurosurgeons to effectively correlate preoperative images with the patient’s physical anatomy during the operation. Imageguidance technology allows the use of “frameless stereotaxy,” offering accuracy similar to that of stereotactic neurosurgery while obviating some of the limitations.9–10
This chapter reviews the use, advantages, and limitations of frameless image guidance for neurosurgical treatment of brain tumors using the optical digitizer. Image guidance for biopsy and minimally invasive resection of brain tumors is reviewed as is the use of this technology for the administration of adjuvant treatment. Finally, the steps of these procedures as conducted at the authors’ institution are described.
 Needle Biopsy
Needle Biopsy
Frameless, image-guided surgery can be successfully used for brain needle biopsy previously performed with frame-based stereotaxy. As part of the initial evaluation for an intracranial mass, patients may undergo stereotactic biopsy, given that numerous studies indicate that presumptive diagnoses based on preoperative clinical information and imaging studies alone are incorrect in as many as 25% of cases. With the use of image-guided techniques the surgical risk of biopsy has significantly declined. Therefore, empirically prescribed cytotoxic therapy with radiation or chemotherapy is usually no longer justified. Image-guided brain needle biopsy, because of its lesser risk, higher yield, and greater cost effectiveness, has superseded the former practice of performing an open craniotomy for biopsy.11
A hallmark of malignant gliomas is heterogeneity. Thus, careful planning is imperative to achieve satisfactory results. This includes tissue diagnosis and tissue sampling that are representative of the whole tumor. A typical example is that of a primary brain tumor nonenhancing after contrast on magnetic resonance (MR) images except in one or multiple small areas. Studies correlating imaging and histology have shown that the enhancing areas most likely contain tumor cells with anaplastic features and possibly of higher tumor grade.12 Obtaining serial biopsies through the bulk of the tumor in a stepwise fashion increases the likelihood of accurate diagnosis. To that end, computer-assisted treatment planning programs are invaluable in selecting a safe and effective trajectory for biopsy. Frozen section analysis provides reassurance of a diagnostic biopsy prior to ending the procedure but should never be considered as sufficient to guide further therapy.
Fusion of positron emission tomography (PET) and MR spectroscopy (MRS) images has been reported to be useful in several areas of neurosurgery.13 In most imageguided systems commercially available, these images can be fused to the routine preoperative MR images. This feature offers a promising role for MRS and especially for PET to enhance the intraoperative accuracy of image-guided stereotactic biopsy by intraoperatively guiding the biopsy to the regions of maximal metabolic rate and presumably highest tumor grade.
When planning a routine diagnostic biopsy a few technical aspects must be remembered. In particular, it is important to acknowledge that the radiographic appearance of glioblastomas and metastasis in some cases is characterized by a ring-enhancing appearance after contrast on preoperative images. The center of the lesion may consist of fluid or necrosis. In either case, when targeting the lesion, care should be taken to avoid the center of the lesion as the first or only target. If fluid is present within the lesion, drainage of it will cause brain shifting, and any additional biopsy at the same setting to obtain diagnostic tissue will most likely be unsuccessful. On the other hand, if necrotic tissue is obtained, it would not be diagnostic. Thus we advocate targeting the periphery first.
The senior author performs all diagnostic biopsies using the frameless equipment as previously reported.11 Exceptions include brain stem biopsies when using a supratentorial approach and biopsy of lesions less than 1.5 cm in maximum longitudinal diameter.
 Minimally Invasive Craniotomy and Open Resection
Minimally Invasive Craniotomy and Open Resection
Intraoperative navigation allows the surgeon to locate a minimally invasive craniotomy flap, thereby reducing the size of the opening and optimizing the center of the craniotomy window. It is important to stress that endoscopy and ultrasound lack this important first step in preparing for resection of a brain tumor.
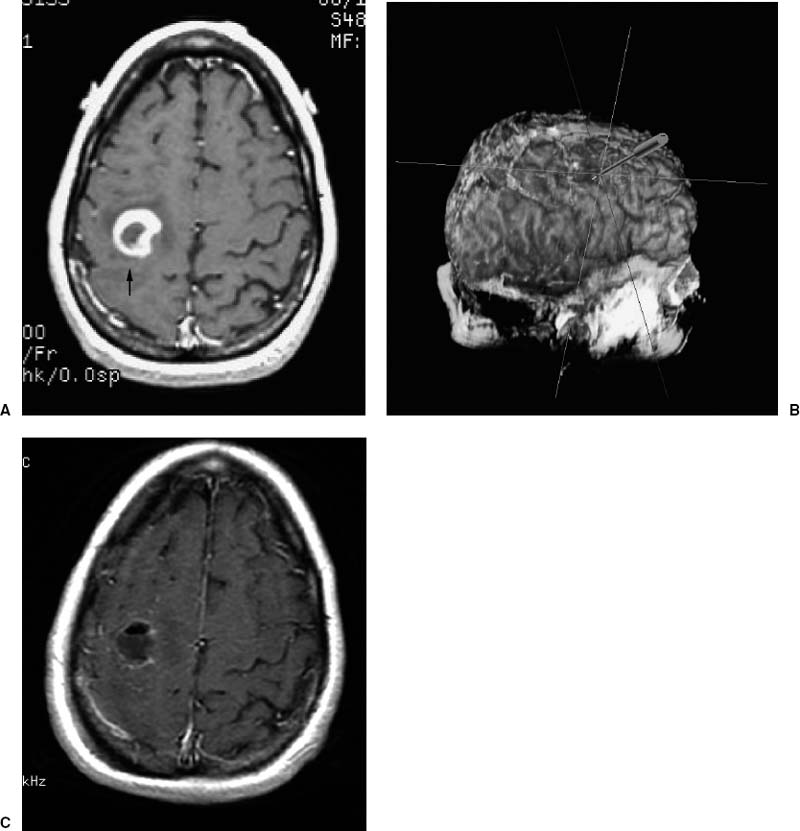
Although the benefit of aggressive resection of primary brain tumors, particularly high-grade malignancies, remains controversial, there is increasing clinical evidence that “complete” resections can prolong the patient’s quality of life and extend survival.2–8 It should be emphasized that when talking about volumetric resection or gross-total resection of a primary brain tumor, this is pertinent only to the “bulk” of the tumor. It is well known that gliomas have infiltrative cells beyond the limit of the resection that require adjuvant treatment in addition to surgery. Thus the image-guided navigator is helpful in allowing volumetric resection of the “ringenhancing” lesion, typical of high-grade gliomas (Fig. 15–1), or the circumscribed area of “decreased signal intensity,” typical of low-grade gliomas on MR images (Fig. 15–2).
Prior to beginning the operation, we perform “virtual surgery” on the computer screen. This step is usually conducted during the induction of anesthesia. When planning for resection of gliomas it is very important to have a precise understanding of the cortical eloquent areas surrounding the tumor (Fig. 15–3). In most cases, eloquent areas such as Broca’s area and motor and sensory cortex can be visualized by the help of the reconstructed triplanar and 3-D images14 (see Figs. 15–1B and 15–2B). Additionally, functional MRI can be fused to the anatomical views to increase the accuracy of functional localization. Although in our experience image guidance provides a very accurate identification of eloquent areas, we confirm our preoperative impression with intraoperative electrophysiological cortical mapping (see Fig. 15–3).
After the craniotomy flap is elevated and prior to beginning the corticotomy, it may be appropriate to perform cortical mapping with bipolar electrical stimulation. Intraoperative motor mapping may be used to minimize the risk of transversing the primary motor cortex. More importantly, functionally eloquent regions of the secondary motor associational cortex can be identified intraoperatively using mapping techniques. The phase reversal seen in somatosensory-evoked potential recordings obtained from electrode arrays placed across the sensorimotor cortex is also very helpful in determining eloquent sensory-motor areas (see Fig. 15–3). Additionally, this technique does not require the patient’s cooperation, and it is less time-consuming. Visual-evoked responses are occasionally employed to variable effect. Expressive language is tested using counting, alphabetical naming, and sentence-generation tests.