Image Quality Assurance Programs
Jeffrey P. Kanne
LEARNING OBJECTIVES
1. Describe modality-specific image quality management strategies
2. State methods for integrating quality management into radiologists’ workflow
3. List the steps of the medical imaging chain and state the relevant stakeholders of each step
Image quality is a critical component of diagnostic medical imaging because suboptimal quality can result in error and uncertainty in interpretation.1,2 Image quality control (QC) and quality assurance (QA) have been longstanding parts of the practice of radiology and primarily have been the responsibilities of the radiology technologist. However, radiologist involvement in image quality management remains critical to ensure that examinations are acceptable for interpretation, and ultimately medical decision making.
IMAGE QUALITY MANAGEMENT
Before the widespread adoption of digital imaging, QC and QA for image quality fell primarily on radiology technologists. In the era of film-screen radiography, dark rooms, processing equipment, and film quality had to be rigorously maintained. A radiology department typically employed a senior technologist to oversee the QC/QA process. This individual would provide feedback to technologists when studies were suboptimal because of exposure, positioning, artifact, or processing. This oversight helped ensure that examinations submitted to radiologists for interpretation were of acceptable and of consistent quality. Additionally, the QC/QA lead served as the liaison between radiologists and technologists. However, as digital radiography and picture archiving and communications systems (PACS) became standard in the practice of radiologist, interactions between technologists and radiologists have waned, both as a result of higher throughput and subsequent increased volumes, as well as distributed imaging studies and resultant increased physical distances between radiologists and technologists. Therefore, the paucity or absence of opportunities for radiologists and technologists to confer on issues of image quality can been seen as lost opportunities for engaging in image QA programs.3
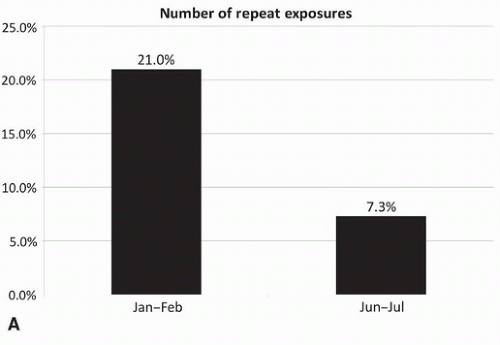
Monitoring image quality and repeat rates should be a regular practice in a radiology department (Fig. 5.1 A-C).4 With film-screen radiography, rejected films could easily be gathered and analyzed for QC purposes (as well as to reclaim silver on the film).5 However, because of the widespread adoption of digital radiography over the past decade, tracking repeat rates has become more complicated. Technologists can simply delete images that they deem inadequate. Additionally, the impetus to track repeat rates has waned with disappearance of the cost of film. Furthermore, the transition from film screen to digital radiography was also associated with a documented decrease in repeat rates because incorrect exposure became less of an issue,5,6,7,8 and with fewer suboptimal studies related to exposure, positioning becomes the primary reason for a repeat acquisition.8 Nevertheless, because of the ease with which technologists can simply discard a suboptimal image and acquire
a new one, there is potential for repeat rates to rise, especially as a younger generation of technologists who train exclusively on digital equipment enters the workforce.
a new one, there is potential for repeat rates to rise, especially as a younger generation of technologists who train exclusively on digital equipment enters the workforce.
The American Association of Physicists in Medicine (AAPM) first published QA guidelines for radiography in 1977,9 followed by updated guidelines in 2002.10 The guidelines advocate for instituting a QC program designed to ensure that radiography studies were obtained with acceptable image quality. The QC program should focus on equipment, processing, and technologist performance. Specifically, the AAPM states in the 2002 report that the repeat rate analysis should be performed at least quarterly, preferably by the same individual to ensure consistency in analysis. To understand repeat rate data, the AAPM suggests that the report contain the following data points: patient positioning, patient motion, artifacts, film fog (for film screen), equipment malfunctions, over- or underexposed films, examination room, technologist, and the anatomical view.
Image quality management extends beyond radiography into other imaging modalities such as computed tomography (CT),
ultrasound (US), magnetic resonance imaging (MRI), fluoroscopy, mammography, and nuclear medicine. Radiologists may be more directly involved in image QA and QC for these modalities because of the lower volumes as compared with radiography, close proximity to technologists such as with US and mammography (especially diagnostic mammography), and extensive involvement in protocol development such as in CT and MRI.
ultrasound (US), magnetic resonance imaging (MRI), fluoroscopy, mammography, and nuclear medicine. Radiologists may be more directly involved in image QA and QC for these modalities because of the lower volumes as compared with radiography, close proximity to technologists such as with US and mammography (especially diagnostic mammography), and extensive involvement in protocol development such as in CT and MRI.
Computed Tomography
Image quality management in CT not only ensures that image quality is adequate but also can help protect patients from unnecessarily high exposures to ionizing radiation. CT operators typically work with medical physicists and vendors to ensure that equipment is functioning properly and scheduled maintenance is performed. Specific features of image quality that require assessment, typically by a trained medical physicist, include CT number, image noise, contrast-to-noise ratio, and spatial resolution.11
The radiologist also plays an important role in CT image quality management. CT protocols are typically developed or modified by radiologists to reflect practice preferences and specific clinical indications. A radiologist or group of radiologists should review CT protocols regularly to confirm that the protocols are still appropriate for the designated clinical indication(s) and that they meet the needs of interpreting radiologists while minimizing patient exposure to ionizing radiation. Additionally, radiologists should regularly monitor scans for higher-than-expected radiation exposure, artifacts, appropriate patient positioning and scan range, and adherence to prescribed protocols.
Ultrasound
Quality management in the United States can be challenging because the US units are becoming increasingly common in hospitals, medical centers, and private offices, and not all US equipment falls under the purview of radiology. The first step in developing an US quality management program is to inventory all ultrasound equipment, including transducers and handheld units. Second, equipment needs to be evaluated by a medical physicist for performance. Specific tests include system sensitivity, uniformity, spatial accuracy, and contrast and spatial resolution. Additionally, the medical physicist should work closely with users of the equipment to ensure that settings are optimized for image quality. Aside from image quality management, US equipment should be evaluated by an appropriate individual such as a clinical engineer for mechanical and electrical safety.12
Magnetic Resonance Imaging
Because of the complexities of MRI, image QC requires substantial technical expertise. The American Association of Physicists in Medicine has published AAPM Report No. 28 “Quality Assurance Methods and Phantoms for Magnetic Resonance Imaging” to “describe a standard set of test procedures, which can be used to evaluate the performance of clinical magnetic resonance imaging systems.”13 Not only must MR units themselves be evaluated, but coils used in clinical scans must also be evaluated. As with CT, a specialized medical physicist is typically delegated the responsibility for managing MRI image quality management.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree