“Knowledge is power…knowledge is safety…knowledge is happiness.” —Thomas Jefferson
Bone lesions are a frequently encountered diagnostic challenge faced by radiologists in the evaluation of pediatric and adolescent patients. Up to 42% of all bone lesions are detected in the first two decades of life, including benign and malignant neoplasms. Nine percent of bone lesions occur in the first decade of life, and approximately 33% in the second.
More than half of all childhood bone neoplasms are benign, with metastatic neuroblastoma being the most common malignant bone lesion occurring in the first two decades of life. The radiologist’s primary goal when encountering a bone lesion in a radiographic examination of a pediatric or adolescent patient is to generate a succinct, logical differential diagnosis based on the imaging characteristics and available clinical information.
A critical component for the generation of an accurate differential diagnosis is distinguishing aggressive osseous lesions from benign “do not touch” lesions and tumor-like lesions, which can be reactive or developmental in etiology.
Aggressive primary bone neoplasms are rare in infants and toddlers but account for a significant proportion of childhood malignancies. The most common primary malignant bone lesions in school-aged children and adolescents are osteosarcoma and Ewing sarcoma (ES). These aggressive pediatric bone malignancies require further evaluation with cross-sectional imaging, as well as image-guided or surgical biopsy.
The most common true benign bone neoplasms in children and adolescents are osteochondroma, osteoid osteoma, osteoblastoma, enchondroma, chondromyxoid fibroma (CMF), and chondroblastoma.
The most common benign tumor-like bone lesions in childhood and adolescence are fibroxanthomas (fibrous cortical defect, nonossifying fibroma [NOF]), simple (unicameral) bone cyst (UBC), aneurysmal bone cyst (ABC), distal femoral cortical irregularity, fibrous dysplasia (FD), and Langerhans cell histiocytosis (LCH).
Radiographs remain the primary imaging modality for the initial evaluation of pediatric bone lesions. Radiographs provide several key characteristics of a lesion, including: (1) a lesion’s location within the host bone; (2) lesion-host bone interface/zone of transition; (3) host bone reaction to the lesion; (4) lesion matrix production; and (5) potential cortical destruction and resulting secondary soft tissue mass.
These key radiographic imaging features, in conjunction with the patient’s chronological age, remain the cornerstones in the differentiation of aggressive and nonaggressive pediatric bone lesions and tumor-like conditions, as well as the generation of an accurate and concise differential diagnosis.
The correct radiographic interpretation of pediatric bone lesions is important because classic benign, nonaggressive pediatric osseous lesions require neither additional cross-sectional imaging work-up nor subsequent biopsy.
Many aggressive and nonaggressive bone neoplasms occur in specific age groups. In addition, certain pediatric osseous lesions frequently occur in characteristic host bones of the axial and appendicular skeleton, as well as specific segments within these host bones. Therefore the patient’s chronological age, host bone, and lesion location within the particular affected host bone are critical factors in the imaging evaluation of pediatric and adolescent bone lesions.
Additional clinical information can also prove invaluable in the imaging evaluation of bone lesions in young patients. The presence or absence of fever, white blood cell count, differential, and erythrocyte sedimentation rate (ESR) are also important pieces of clinical information that can assist in honing the differential diagnosis of a pediatric bone lesion.White blood cell count and ESR are often elevated in the setting of osteomyelitis but can also be elevated in acute leukemia, lymphoma, ES, and LCH.
Cross-sectional imaging with magnetic resonance imaging (MRI) and/or computed tomography (CT) can provide additional valuable imaging evaluation of a bone lesion if the diagnosis remains uncertain after the initial radiographic examination. MRI evaluation is critical for the evaluation of potential skip lesions, soft tissue extension, integrity of adjacent neuromuscular structures, initial staging, and preoperative planning for malignant osseous lesions. CT, with its high spatial resolution, can provide additional information regarding a lesion’s potential internal matrix production, associated cortical erosion/disruption, or risk for potential pathological fracture.
Nuclear medicine bone scintigraphy also has a role in the evaluation of metastatic disease and/or multifocal processes, but these examinations are nonspecific. Bone scans are positive for lesions that stimulate reactive new bone formation when technetium-99m-methylene diphosphonate (MDP) is adsorbed onto newly incorporated hydroxyapatite. Lytic lesions, such as LCH, associated with bone destruction are better evaluated with radiographic skeletal surveys. Positron emission tomography (PET)-CT and MIBG (metaiodobenzylguanidine) nuclear medicine scintigraphy are additional useful modalities for the staging and posttherapy surveillance imaging of neuroblastoma.
The goal of this chapter is to put forth a systematic imaging approach for the generation of an accurate, concise differential diagnosis for pediatric and adolescent bone lesions based on the combination of characteristic imaging features of these lesions and vital corroborative clinical information.
In addition, this chapter aims to assist the radiologist in recommending the most effective imaging work-up of pediatric and adolescent bone lesions, as well as finalizing the diagnosis and defining the extent of disease.
Systematic Approach
The proper imaging evaluation of pediatric bone lesions requires answering the following diagnostic questions ( Box 14.1 ).
- 1.
What is the age of the patient?
- 2.
What is the involved host bone?
- 3.
What segment of the host bone is affected?
- 4.
What is the host bone reaction to the lesion?
- 5.
Is the lesion unifocal or part of a multifocal process?
Patient Age
The chronological age of the affected patient is a vital piece of information in the imaging evaluation of pediatric bone lesions. Osseous lesions demonstrate a propensity for affecting certain age ranges in young patients. Establishing the age of the affected child is extremely helpful in beginning to formulate an accurate differential diagnosis.
The age ranges that have been used in the imaging evaluation of pediatric bone lesions are: infants and toddlers (<5 years of age), children (5–10 years of age), and adolescents (10–20 years of age). Specific bone lesions demonstrate peak occurrence rates within these age ranges. Thus the chronological age of an affected patient is critical in narrowing the differential diagnosis of an encountered bone lesion ( Box 14.2 ).
Infants and toddlers: <5 years of age
Children: 5–10 years of age
Adolescents: 10–20 years of age
Infants and Toddlers (<5 Years of Age)
Primary bone malignancies are extremely rare in infants and toddlers. For example, ES is rarely encountered before the age of 5 years. The differential diagnoses of aggressive bone lesions in infants and toddlers are acute leukemia, multifocal LCH, metastatic lesions secondary to neuroblastoma, and osteomyelitis ( Box 14.3 ).
Acute leukemia
Multifocal Langerhans cell histiocytosis
Metastatic lesions secondary to neuroblastoma
Osteomyelitis
Children (5–10 Years of Age)
The differential diagnoses for aggressive bone lesions in children (5–10 years old) are ES, monostotic LCH, and osteomyelitis ( Box 14.4 ).
Ewing sarcoma
Monostotic Langerhans cell histiocytosis
Osteomyelitis
Adolescents (10–20 Years of Age)
The differential diagnoses for an aggressive osseous lesion in adolescents (10–20 years of age) are osteosarcoma, ES, leukemia, primary lymphoma of bone (PLB), and osteomyelitis ( Box 14.5 ).
Osteosarcoma
Ewing sarcoma
Acute leukemia
Primary lymphoma of bone
Osteomyelitis
The differential diagnoses for nonaggressive osseous lesions in the adolescent age range (10–20 years of age) are extensive, including fibroxanthoma (fibrous cortical defect, NOF), osteochondroma, UBC, ABC, chondroblastoma, FD, osteoid osteoma/osteoblastoma, enchondroma, CMF, and periosteal/juxtacortical chondroma ( Box 14.6 ).
Fibroxanthoma
Osteochondroma
Simple/Unicameral bone cyst
Aneurysmal bone cyst
Chondroblastoma
Fibrous dysplasia
Osteoid osteoma/Osteoblastoma
Enchondroma
Chondromyxoid fibroma
Periosteal/Juxtacortical chondroma
Host Bone
Pediatric bone lesions demonstrate a pattern of occurrence within specific host bones. Bone neoplasms and tumor-like lesions have a propensity for development within regions of rapid bone growth and remodeling. Thus lesions tend to occur near the ends of long tubular bones in growing patients. For example, osteosarcoma commonly develops within the metaphyses of the distal femur and proximal tibia in the region of the knee. Osteosarcoma also occurs within the proximal metaphyses of the humerus, which are all regions of rapid bone growth.
Hemangiomas commonly occur in vertebral bodies and are the most common radiolucent lesion of the spine. Other osseous lesions also demonstrate a proclivity for occurring in the spine, such as osteoid osteoma, osteoblastoma, and ABC. However, these lesions occur within the posterior vertebral elements ( Box 14.7 ).
Vertebral Body
Hemangioma
Metastasis
Lymphoma
Posterior Vertebral Elements
Osteoid osteoma/Osteoblastoma
Aneurysmal bone cyst
Radiolucent lesions can occur in the calcaneus of young patients, an epiphyseal equivalent. The differential diagnosis for a radiolucent calcaneal lesion in a young patient includes a UBC, ABC, chondroblastoma, giant cell tumor (GCT), and pseudolesion ( Box 14.8 ).
Simple/Unicameral bone cyst
Aneurysmal bone cyst
Chondroblastoma
Giant cell tumor
Pseudolesion
Intraosseous lipoma
FD, LCH, ES, lymphoma, and osteosarcoma can develop in the ribs of young patients ( Box 14.9 ). However, a healing fracture must be considered in the formulation of a differential diagnosis of any pediatric rib lesion.
Metastasis
Langerhans cell histiocytosis
Fibrous dysplasia
Ewing sarcoma
Lymphoma
Osteosarcoma
Longitudinal Plane of the Host Bone
Pediatric and adolescent bone lesions also typically occur within certain regions along the longitudinal axis of the host bone ( Fig. 14.1 ).

For example, chondroblastomas classically occur in the epiphysis and epiphyseal equivalents of long bones. Rarely, epiphyseal osteochondroma-like lesions can occur within long bones in the setting of Trevor-Fairbank disease (dysplasia epiphysealis hemimelica) ( Box 14.10 ).
Osteomyelitis (<18 months of age)
Chondroblastoma (open physis)
Giant cell tumor (closed physis, extending from the metaphysis)
Osteoid osteoma (Trevor-Fairbank disease)
Benign UBCs and ABCs, as well as osteochondroma and enchondroma, most commonly occur in the metaphases of long bones. These nonaggressive lesions can demonstrate subsequent migration away from the physes as young patients continue to grow.
GCTs, which can occur in adolescents and young adults, are classically centered in the metaphysis of long tubular bones with subsequent extension into the epiphysis after physeal closure. Osteomyelitis most commonly occurs in the metaphysis of long bones because of the presence of stagnant, slow-flowing blood within multiple looping blood vessels and sinusoids in this region.
Osteosarcoma most commonly occurs in the metaphysis of long bones as well. Therefore the differential diagnosis of an aggressive metaphyseal lesion in a long tubular bone in the second decade of life should include infection and osteosarcoma. Benign fibroxanthomas (fibrous cortical defect/NOFs), as well as CMFs, classically occur in the metaphysis and diametaphysis of long bones ( Box 14.11 ).
Benign
Fibroxanthoma: Fibrous cortical defect/nonossifying fibroma
Enchondroma
Simple/Unicameral bone cyst (central)
Aneurysmal bone cyst (eccentric)
Osteochondroma
Osteomyelitis
Giant cell tumor (extending into the epiphysis if physis is closed)
Osteoid osteoma
Malignant
Metastasis
Primary lymphoma of bone
Osteosarcoma
FD, osteofibrous dysplasia (OFD), and enchondromas can occur in the diametaphysis of long bones. FD and OFD also can occur in the diaphysis of long bones. Small round blue cell malignancies, such as PLB, acute leukemia, and ES, also occur in the diametaphysis and diaphysis of long bones ( Box 14.12 ).
Axial Plane of the Host Bone
Pediatric bone lesions also demonstrate patterns of occurrence with respect to the axial plane of the host bone. Lesions can be located centrally or eccentrically within the medullary cavity of the host bone. In addition, lesions also can occur within cortical or juxtacortical locations relative to the axial plane of the host bone. Notably, periosteal lesions arise from the deep (cambium) layer of the periosteum and separate the periosteum from the subjacent cortex. Parosteal lesions arise from the outer (fibrous) layer of the periosteum and grow exophytically without elevating the subjacent periosteum from the cortex.
The differential diagnosis of an intramedullary lesion can therefore be narrowed by the identification of the epicenter of these lesions. Central intramedullary lesions of long bones include enchondroma, FD, and UBC ( Box 14.13 ).
Enchondroma
Fibrous dysplasia
Simple/Unicameral bone cyst
Eccentric intramedullary lesions include NOF, CMF, ABC, and GCT ( Box 14.14 ).
Nonossifying fibroma
Chondromyxoid fibroma
Aneurysmal bone cyst
Giant cell tumor
Cortically based lesions include fibrous cortical defect and osteoid osteoma ( Box 14.15 ). Juxtacortical lesions include osteochondroma, periosteal/juxtacortical chondroma, as well as periosteal and parosteal osteosarcoma, which are rare in young patients ( Box 14.16 ).
Fibrous cortical defect
Osteoid osteoma
Periosteal/Juxtacortical chondroma
Osteochondroma
Host Bone Reaction
The evaluation of the type of periosteal reaction and periosteal new bone formation, or lack thereof, generated by a host bone in response to the presence of a bone lesion can assist the radiologist in narrowing the differential diagnosis. Periosteal reaction is a nonspecific response by the host bone to the underlying lesion. As the periosteum is irritated, and potentially raised or disrupted by a bone lesion, the host bone forms new bone in an attempt to contain the lesion.
Pediatric and adolescent bone lesions incite relatively early periosteal reactions because the periosteum in young patients demonstrates looser attachment to the underlying host bone in comparison with adults. Aggressive and nonaggressive osseous lesions demonstrate a high degree of overlap in the morphology of periosteal reactions they elicit. Of note, prior studies have demonstrated aggressive osseous lesions can result in benign or no periosteal reactions. Conversely, aggressive morphologies of periosteal reaction do not confirm the presence of an underlying aggressive malignancy.
In general the morphology of the resulting periosteal reaction and the density of the periosteal new bone formation, although nonspecific, are indicators of the rate of growth of the inciting osseous lesion. Slowly expanding, nonaggressive bone lesions typically result in a lack of periosteal reaction or dense, thick periosteal new bone formation from the host bone. Benign forms of periosteal new bone formation can demonstrate a continuous single layer or solid morphologies ( Fig. 14.2 ).

Rapidly growing, aggressive bone lesions classically result in lower-density periosteal new bone formation because the parent bone has less time to lay down new bone to contain a rapidly expanding lesion. Aggressive morphologies of periosteal reaction are spiculated in perpendicular hair-on-end and radial sunburst patterns. These forms of periosteal reaction are due to periosteal new bone formation along innumerable Sharpey fibers, which connect the periosteum to the subjacent cortex.
Lamellated or “onion skin” and disrupted periosteal new bone formation “Codman triangle” morphologies also occur in the setting of aggressive osseous lesions. Osteosarcoma and ES can result in these types of periosteal new bone formation. Notably, nonmalignant processes such as osteomyelitis and thalassemia also can result in aggressive forms of periosteal reaction ( Fig. 14.3 ).

Legion Margins/Patterns of Bone Destruction
The margin/zone of transition formed between an osseous lesion and the host bone can be helpful to the radiologist in the generation of a differential diagnosis. The type of margin can indicate the lesion growth rate. The pattern of bone destruction and subsequent lesion margin can be described as geographical, moth-eaten, or permeative.
Geographical bone destruction almost always results in well-defined margins and is generally present in the setting of a single discrete lytic lesion that is often well circumscribed with or without a sclerotic border. Geographical lesions tend to be nonaggressive and are almost always benign. Nonaggressive osseous lesions that result in geographical bone destruction include NOFs, bone cysts, enchondromas, FD, and chondroblastomas.
“Moth-eaten” and permeative patterns of bone destruction indicate that a bone lesion is more aggressive and often malignant. Aggressive bone destruction often results in multiple ill-defined lytic defects with poorly defined margins (“moth-eaten” appearance). Permeative bone destruction permeates through the bone and can be difficult to visualize on radiographs; therefore MRI is extremely helpful in the imaging evaluation of a subtle permeative lytic lesion. Aggressive osseous lesions that can result in these forms of bone destruction include osteosarcoma, ES, osteomyelitis, and LCH.
The effect of an osseous lesion on the overlying cortex of the host bone also can be informative in the generation of a differential diagnosis. Cartilaginous lesions such as enchondromas are associated with endosteal scalloping. FD also can result in mild medullary expansion and endosteal scalloping. ABCs can result in prominent endosteal scalloping and cortical expansion.
Matrix and Mineralization
The mesenchymal cells of bone lesions produce the characteristic internal matrices of these neoplasms. The internal matrix of these lesions can be osseous, cartilaginous, fibrous, or myxoid. Bone neoplasms are generally named for the type of internal matrix they produce. Osseous lesions can produce a mixture of matrix patterns, such as FD, which can demonstrate osseous and cartilaginous matrix production. CMF produces both chondroid and myxoid matrix types. In contrast, not all lesions produce a matrix, such as ES and GCTs.
Osseous matrix mineralization results in a cloudlike, fluffy, or ivory density within bone lesions. Lesions producing an osseous matrix can form immature woven bone or mature lamellar bone. Osteosarcoma can produce both types of osseous matrix. FD forms immature woven bone, which is less dense than mature lamellar bone, resulting in the “ground-glass” density characteristic of FD. Chondroid matrix production can result in the classic arc and ring morphology. However, chondroid matrix also can exhibit a stippled or flocculent appearance.
Soft Tissue Component
In general the presence of a soft tissue component associated with an osseous lesion indicates underlying cortical disruption in the setting of an aggressive process. Soft tissue mass components associated with bone lesions can be evaluated for on radiographs by looking for variable densities and effacement of normal tissue planes within the soft tissues adjacent to the affected host bone. In addition, the presence of mineralization and/or matrix production within the juxtacortical soft tissues can indicate the presence of an underlying soft tissue mass component. Follow-up intravenous contrast-enhanced MRI should be performed of a bone lesion if an associated soft tissue mass is suspected.
Single or Multiple Lesions
Multiple osseous lesions in a young patient indicate the presence of an underlying systemic disease or syndrome predisposing to the development of bone lesions. Primary bone neoplasms tend to be monostotic. A young patient demonstrating constitutional symptoms and multiple lytic bone lesions should be considered for acute leukemia, multifocal osteomyelitis, or metastatic neuroblastoma. ES with metastases, primary bone lymphoma (PBL), or multifocal LCH should also be considered depending on the age of the patient. Notably, patients with sickle cell are at increased risk for hematogenously disseminated multifocal osteomyelitis, particularly secondary to encapsulated organisms such as Salmonella .
Multiple lucent “brown tumors” can also be present in the setting of underlying hyperparathyroidism. FD and LCH can demonstrate a polyostotic distribution, as described earlier. Polyostotic FD can occur in the setting of McCune-Albright syndrome (MAS). Multiple enchondromas are present in the setting of Maffucci syndrome or Ollier disease. Multiple NOFs can occur in the setting of neurofibromatosis type I and Jaffe-Campanacci syndrome. Multiple osteochondromas are present in multiple hereditary exostoses (MHE).
Infants and Toddlers (<5 Years of Age)
Acute Leukemia
Acute leukemia is the most common malignancy of childhood, with acute lymphoblastic leukemia representing approximately 75% of cases, and acute myelogenous leukemia representing approximately 15% to 20% of pediatric cases. The peak incidence is between 2 and 10 years of age.
Children present with bone or joint pain, limp, soft tissue swelling, and/or arthralgias. Fatigue secondary to anemia, fever, petechiae, bleeding, and hepatosplenomegaly also can be present at presentation. These children also can demonstrate an elevated ESR, thrombocytopenia, and neutropenia.
Radiographs are often normal or can have subtle findings ( Fig. 14.4A ). Forty percent of pediatric patients with acute leukemia demonstrate radiographic findings. Diffuse osteoporosis can be present or classic radiolucent “leukemic lines,” which occur in the metaphysis of long bones adjacent to the zone of provisional calcification (ZPC), which often remains intact.

Poorly defined osteolytic metaphyseal lesions can occur with a “moth-eaten” or permeative appearance. These lesions can result in aggressive periosteal reactions demonstrating lamellated, spiculated, and/or interrupted morphologies (Codman triangle). Pathological fractures can occur in the setting of these aggressive lesions in the background of osteoporosis. Juxtacortical soft tissue masses can occur secondary to cortical and periosteal disruption.
T1-weighted imaging demonstrates confluent T1-hypointense regions secondary to replacement of normal marrow by leukemic infiltrates ( Fig 14.4B ). This T1-hypointense signal will correlate with patchy or diffuse marrow edema secondary to marrow infiltration. These hypercellular leukemic marrow infiltrates can demonstrate restricted diffusion, as well as intermediate to bright enhancement. Bone scans will demonstrate increased radiotracer uptake in foci of osseous leukemic involvement; however, bone scans may underestimate the extent of disease. PET-CT and whole-body MR can accurately assess for diffuse systemic involvement and treatment response.
Multifocal Langerhans Cell Histiocytosis
LCH is a disorder characterized by clonal proliferation of S100-positive dendritic cells. The current classification of LCH is currently based on disease extent, and may be single-system (unifocal or multifocal) and multi-system, with or without risk organ involvement (spleen, liver, or bone marrow) . Ninety percent of cases of LCH present before the age of 15 years. The peak incidence of multisystem disease is less than 3 years of age
The skeletal system is involved in 80% to 90% of cases, which rarely occurs in a unifocal form in infants and toddlers. Osseous lesions will most commonly involve the calvarium and classically demonstrate well-defined round or lobulated “punched-out” lesions without sclerotic rims ( Fig. 14.5A ). These lesions can exhibit “beveled” edges due to asymmetric involvement of the inner and outer tables of the calvarium. Flat bones are affected most commonly, with the skull, mandible, ribs, pelvis, and scapula being common sites of osseous involvement. Longs bones and vertebrae are affected less commonly by LCH, with vertebral lesions occurring most frequently in the thoracic and lumbar spine.

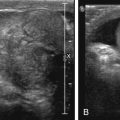
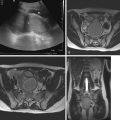
Vertebra plana secondary to a lytic lesion centered within a vertebral body is a classic finding in pediatric LCH spine involvement with secondary kyphosis and/or scoliosis. The adjacent disks and posterior elements are rarely involved. A diminutive soft tissue mass component relative to the degree of vertebral body destruction is characteristic of LCH spinal involvement. The differential diagnosis for a lytic spinal lesion in an infant or toddler with fever and leukocytosis should include acute leukemia, LCH, and osteomyelitis.
LCH skeletal lesions within the appendicular skeleton will demonstrate variable radiographic appearances depending on the phase of disease and subsequent treatment effects. Lesions are more commonly radiolucent than sclerotic and can exhibit geographical or permeative bone destruction with variable margins (see Fig. 14.5B–C ). Aggressive and nonaggressive periosteal reactions can be present in the setting of LCH lesions. In addition, soft tissue masses can occur overlying lytic lesions, simulating an aggressive malignancy. LCH lesions most commonly occur in the metaphyses or diaphyses of long bones (see Fig. 14.5D–E ).
A button sequestrum, a sclerotic focus within a lucent lesion, can occur. In addition, the floating tooth sign is a classic finding in the setting of mandibular involvement of LCH secondary to destruction of the dense lamina dura.
Radiographic skeletal surveys, F-18 fluorodeoxyglucose ( 18 F-FDG)-PET/CT, and whole-body short-tau inversion recovery (STIR) MRI can be used to evaluate for multifocal LCH and monitor treatment response. These osseous lesions demonstrate variable radiotracer uptake with common false negatives on nuclear medicine bone scans which have a lower sensitivity than radiographic skeletal surveys. Notably, these osseous lesions can demonstrate decreased radiotracer uptake with a surrounding halo of increased uptake, particularly in the calvarium.
Whole-body STIR MRI can assess for multifocal osseous and extraskeletal disease. Notably, MRI is more sensitive than radiographs or bone scan in the detection of multifocal lesions. However, STIR imaging is not effective at distinguishing acute from residual disease.
18 F-FDG-PET/CT is highly sensitive for active LCH lesions; however, it is less sensitive than MRI for vertebral involvement. In addition, 18 F-FDG/CT results in a higher radiation dose than the other imaging modalities. This radiation dose can be lowered with the use of lesion-selective CT imaging, as well as the utilization of PET/MR fusion. Thus PET/MR fusion will likely prove to be the most efficacious modality for multifocal lesion detection and treatment monitoring in the future.
Metastatic Neuroblastoma
Neuroblastoma is a malignant neoplasm of sympathetic chain primitive neural crest cells and is the most common extracranial solid malignancy in childhood with a median age at diagnosis of 15 to 17 months. Primary neuroblastoma can occur anywhere along the sympathetic chain from the neck to the pelvis, most commonly within the adrenal glands.
Metastases are present at diagnosis in 50% to 60% of cases of neuroblastoma. The most common sites of metastatic disease are bone, lymph nodes, liver, and soft tissues. Neuroblastoma is the most common primary malignancy resulting in metastatic bone disease in young children.
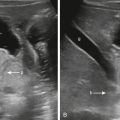
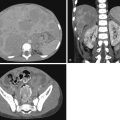
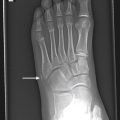
Neuroblastoma osseous metastatic lesions can manifest as focally destructive cortical lesions and/or intramedullary lesions and are the most common cause of aggressive metaphyseal lesions in young children ( Fig. 14.6A–B ). Notably, extensive metastatic marrow disease can be present with little radiographic change.