Acknowledgments
I am grateful to Robert Lebowitz, Rhonda Johnson, and Jane Choura for their invaluable help in preparing this chapter and, always, for their friendship.
Key point: Distinguishing normal from pathological causes of urinary tract dilation (UTD) is one of the most important topics in pediatric uroradiology. Most etiologies of UTD in children are congenital.
Understanding UTD is one of the primary indications for radiological evaluation of the urinary tract in fetuses and children. Most causes of UTD in children are congenital and are first detected in utero. Routine antenatal ultrasound (US) has led to the detection of UTD of 1% to 2% of fetuses. Many of these children are followed after birth. However, 70% to 80% of fetal UTD resolves and requires no further imaging. Thus distinguishing the normal appearance, the abnormal appearance, and the causes of abnormal UTD is an important subject in pediatric uroradiology.
This chapter outlines the normal appearance of the urinary tract, the congenital causes of abnormal UTD, and the tests used in distinguishing the causes and severity of disease. The common treatments, medical and surgical, will also be described so that the radiologist can be familiar with the outcomes of their diagnoses.
Many descriptive terms have been used to describe UTD, including hydronephrosis, hydroureter, hydroureteronephrosis, pyelectasis, pelviectasis, and caliectasis. Grading systems are also used in children that have been popularized by the Society of Fetal Urology, European Society of Pediatric Radiologist, and the Onen system. However, not all of these descriptive systems are applied to fetuses.
In 2014, practitioners in all fields caring for fetuses and infants with UTD formed a new UTD system to try to standardize descriptions in fetuses and infants ( Fig. 8.1 ). This system uses the following criteria to describe and grade the appearance of the urinary tract: anterior-posterior renal pelvic diameter, pelvic dilation, calyceal dilation (with a distinction between central and peripheral calyces), ureteral dilation, bladder appearance, and renal parenchymal appearance. In utero, the amniotic fluid quantity is also an important factor ( ; ; ).

How Do We Evaluate Urinary Tract Dilation?
Key point: US is the screening test of choice of the child’s urinary tract. Additional tests provide functional information.
Ultrasound
US is the screening test of choice of the urinary tract because it is readily available, inexpensive, and emits no ionizing radiation, a particular concern in children. Most postnatal USs to follow up prenatal hydronephrosis are performed in the first month of life, at least 48 hours after birth. Normal newborn oliguria in the first few days of life can cause underdistention of the urinary tract, and the appearance of the urinary tract at that time may falsely underestimate the degree of dilation. If the suspected prenatal abnormality is severe enough to be treated shortly after birth, such as posterior urethral valves (PUVs) in boys, then earlier imaging may be warranted.
The normal kidneys in the newborn period have a characteristic appearance that is different from the adult kidney. The medullary pyramids are hypoechoic, and this should not be confused for dilation of the renal calyces ( Fig. 8.2 ). Later, the hypoechoic pyramids become more homogeneous with the surrounding renal parenchyma, and the kidneys assume a more “adult appearance” ( Fig. 8.3 ). The normal renal parenchyma in a newborn has a variety of appearances. Unlike adults where the echogenicity of the kidney is normally less than that of the liver or spleen, the normal newborn renal parenchyma can be hypoechoic, isoechoic, or hyperechoic relative to the liver or spleen ( Fig. 8.4 ). Normal hyperechoic parenchyma is especially common in newborns and premature infants.



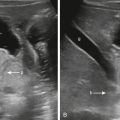
It is important to distinguish a normal amount of fluid within the urinary tract from abnormal quantities of fluid. Because the function of the urinary tract is to drain the urine, fluid is normally present. The normal measurements of the renal pelvis measured in the transverse plane or anteroposterior renal pelvis diameter (APRPD) is normally less than 10 mm. The average measurement increases slightly with age and with a full urinary bladder. When the calyces become dilated, especially peripheral calyces, the dilation becomes more abnormal ( Fig. 8.5 ). The greater the degree of UTD, the more potentially abnormal is the urinary tract. However, a normal urinary tract on US does not exclude pathology, particularly vesicoureteral reflux (VUR; Fig. 8.6 ) ( ).


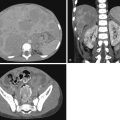
Unless careful attention is paid to the ureters, these structures are frequently not seen by US. Occasionally the normal ureter is seen to be slightly distended by urine because of a normal peristaltic wave. Normal ureteral jets of urine are seen intermittently through the ureteral orifices and into the bladder. A normal ureter measures 4 mm or less in diameter. The more dilated the ureter, the more likely it is that there is an abnormality causing the dilation ( ; ; ) ( Fig. 8.7 ).

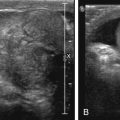
Lower urinary tract abnormalities can lead to hydroureteronephrosis. For example, boys with urethral obstruction caused by PUVs may have UTD from either reflux or ureterovesical junction obstruction in the presence of an incompetent ureteral orifice. Reflux is caused by increased intravesical pressure. The same pressure over time also causes bladder wall hypertrophy, which can result in ureterovesical junction obstruction. Occasionally the ureter may both reflux and be obstructed. Often the effect of bladder outlet obstruction is different in one ureter than the other. Thus carefully examining the bladder or urethra is crucial to understanding the cause of UTD. A normal bladder wall is smooth ( Fig. 8.8 ). When hypertrophy occurs, the bladder wall becomes trabeculated ( Fig. 8.9 ).


The goals of imaging are not only to visualize the urinary tract but also to help determine which conditions are pathological and may result in deterioration of renal function. The greater the degree of UTD, the more likely it is that there is an abnormality that is causing it, typically obstruction.
After an US determines that the urinary tract is abnormally dilated, additional tests (dynamic renal scintigraphy, cortical scintigraphy, magnetic resonance urography [MRU], fluoroscopic voiding cystourethrography, and radionuclide cystography) distinguish obstructed urinary tracts from those with reflux and measure the degree of renal functional impairment. When ultrasound contrast agents are injected intravenously (IV) or infused intravesically, US can serve as a functional study as well.
Dynamic Renal Scintigraphy
Dynamic renal scintigraphy and diuresis renography are commonly used tests to assess UTD to help assess UTD by assessing the degree of obstruction. Dynamic renal scintigraphy is performed with the radiopharmaceutical 99m Tc-mercaptoacetyltriglycine ( 99m Tc-MAG3), which is excreted by active renal tubular transport. 99m Tc-diethylene-triamine-pentacetic acid is a less commonly used alternative to 99m Tc-MAG3.
Dynamic renal scintigraphy depends on the rapid excretion of radiopharmaceuticals through the kidney. This requires three steps: (1) renal perfusion and cortical uptake of 99m Tc-MAG3, (2) cortical transit of 99m Tc-MAG3 into the renal collecting system, and (3) excretion of 99m Tc-MAG3 through the urinary collecting system. Imaging and quantitative measurement of each of these steps provides information about renal function. The pattern of cortical uptake, which is measured during the first 2 minutes of the study, can identify regions of cortical hypoperfusion or scar, as well as provide a measure of differential (left versus right) renal function. Although this provides approximate differential function, a renal cortical scan (typically dimercaptosuccinic acid (DMSA)) provides more accurate indication of differential renal function. The rate of cortical transit, normally 3 to 6 minutes, is an indicator of renal function. The rate and pattern of 99m Tc-MAG3 excretion is used to assess collecting system drainage. Delayed drainage may indicate collecting system obstruction, but also may occur with low urine flow or in a markedly dilated collecting system. With diuresis renography, urine flow is increased, typically with IV saline infusion and IV administration of furosemide, to help distinguish collecting system obstruction from other causes of delayed collecting system drainage ( Fig. 8.10 ).


Several patient factors can confound interpretation of the studies. Infants, especially those younger than 1 month, have functionally immature kidneys, which may delay cortical uptake and excretion of radiopharmaceuticals. In older children, impaired renal function can slow urinary excretion of radiopharmaceutical and limit evaluation of the urinary collecting system. Recent administration of radiographic contrast may transiently diminish radiopharmaceutical excretion.
Cortical scintigraphy does not evaluate UTD directly, but it does measure functioning renal tissue. The amount of functional renal tissue is an important parameter in helping to determine the management of the child.
Magnetic Resonance Urography
MRU has the combined benefit of clearly depicting anatomic structures, showing the uptake and excretion of contrast through the urinary tract over time, and calculating parameters to show differential renal function. To assess function, like the diuretic renography, the patient needs an IV line for hydration, contrast administration, and a diuretic challenge. The main limitation of this technique is the need to keep the patient still for the relatively lengthy examination (45 minutes). This may necessitate sedation.
Static noncontrast T1 and fluid-sensitive sequences depict the anatomic appearance of the urinary tract beautifully in three dimensions. The fluid-sensitive sequences show dilation of the urinary tract and clearly show the sites and causes of obstruction ( Fig. 8.11A ).

During dynamic imaging with contrast enhancement, the perfusion, concentration, and excretion by the kidney can be seen and measured through changes in signal intensity over time. The differential renal function between the kidneys is measured by the degree of contrast enhancement. The Patlak number, an indirect measure of glomerular filtration rate, is a surrogate for renal function and can be measured by MRU. This study also has the advantage of showing the anatomy of the renal vessels, including crossing vessels, which may lead to ureteropelvic junction obstruction (UPJO; see Fig. 8.11B ).
MRU is helpful in predicting which patients may regain renal function after surgery. If a dilated urinary tract shows normal drainage after hydration and administration of a diuretic, the system is considered compensated. In kidneys with compensated hydronephrosis, surgery does little to improve the function of the kidney. However, if high flow rates result in inadequate drainage of the urinary tract during MRU, the system is considered decompensated. In these patients, successful surgery will improve the function of the kidney.
Tests for Vesicoureteral Reflux: Voiding Cystourethrogram, Radionuclide Cystogram, and Contrast-Enhanced Voiding Urosonography
VUR occurs when there is abnormal retrograde flow of urine from the bladder up into the ureter, renal pelvis, or calyces. Reflux, especially high-grade reflux, can cause UTD. Three tests are available to evaluate for reflux: the fluoroscopic voiding cystourethrogram (VCUG), the radionuclide cystogram (RNC), and the contrast-enhanced voiding urosonography (ceVUS) ( Fig. 8.12 ). Each of these tests is performed similarly but use different contrast agents.