End-stage organ failure is commonly treated with transplantation of the respective failing organ. Although outcomes have progressively improved over the decades, early and late complications do occur, and are often diagnosed by imaging. Given the increasing survival rates of transplant patients, the general radiologist may encounter these patients in the outpatient setting. Awareness of the normal radiologic findings after transplantation, and imaging findings of the more common complications, is therefore important. We review and illustrate the imaging assessment of complications from lung, liver, and renal transplantation, highlighting the key similarities and differences between pediatric and adult patients.
Key points
- •
The diseases leading to solid organ failure differ between children and adults; however, similar postoperative complications are seen after transplantation.
- •
Children in general have higher rates of many types of postoperative complications following solid organ transplantation.
- •
Differences in surgical technique in the pediatric population, including use of downsized grafts, lead to varied postoperative anatomy and imaging appearance compared with adults.
- •
Posttransplant malignancies are an increasingly important consideration as overall posttransplant survival rates improve and pediatric transplant patients age into adulthood.
Introduction
Solid organ transplantation is performed for end-stage organ disease in both children and adults. Overall, similar types of complications are seen between the 2 populations; however, children’s small size can impact surgical methods, as well as the relative frequency of complications, particularly vascular complications. Adolescents have on average poorer outcomes posttransplant, likely due to difficulties with medication compliance. In addition, the different etiologies leading to end-organ failure and transplantation between the 2 populations have some bearing on the postoperative imaging appearance. As there are relatively few transplant centers, many patients travel considerable distances to receive their transplants. Travel back to these transplant centers for routine follow-up is often not feasible. Thus, the general radiologist may encounter these patients in their practice. As clinical presentations are frequently nonspecific or asymptomatic, it is essential that general radiologists recognize complications of common solid organ transplants on imaging, particularly in the intermediate to delayed time frame.
This article discusses the imaging appearance of lung, kidney, and liver transplants in children and adults, with a focus on imaging techniques, normal radiological appearance, and complications specific to graft type. In addition, this article also reviews the imaging diagnosis of postoperative fluid collections and posttransplant lymphoproliferative disease (PTLD), which may be seen with all 3 types of transplantation.
Lung transplantation
Lung transplantation is relatively infrequent in children compared with adults, and is performed much less frequently in children than heart, liver, or kidney transplants. However, as there are relatively few lung transplant centers, particularly for children, routine follow-up of these patients may occur in the community rather than at the transplant center. Therefore, general radiologists must be familiar with findings concerning for intermediate and particularly late complications of lung transplantation.
Underlying diseases leading to lung transplantation differ between pediatric and adult populations. In children, pulmonary hypertension constitutes the most common illness requiring lung transplantation in 1-year-olds to 5-year-olds, whereas cystic fibrosis accounts for the overwhelming majority in 6-year-olds to 11-year-olds. , Combined heart-lung transplantation is now rarely performed, predominantly for idiopathic pulmonary hypertension. In contrast to children, the most common underlying cause in adults is fibrotic lung disease, such as idiopathic pulmonary fibrosis, followed by obstructive disease, such as emphysema.
Surgical Techniques
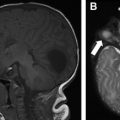
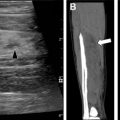
The chief difference in surgical technique between pediatric and adult lung transplants revolves around donor-recipient size mismatch. Size mismatch may occur in both the pulmonary parenchymal size, and the bronchial anastomotic size. The relative paucity of pediatric donors leads to the use of downsized adult donor lungs in pediatric recipients. Lungs may be downsized via lobectomies or with a nonanatomic reduction using a linear stapling device ( Fig. 1 ). Oversized pulmonary transplants may manifest as atelectasis on postoperative radiographs. Conversely, undersized pulmonary transplants may lead to chronic pleural effusion or pneumothorax due to residual unfilled thoracic cavity ( Fig. 2 ).


Imaging Techniques and Normal Findings
Chest radiographs are most commonly used for the initial postoperative evaluation of lung transplants, as they are portable, fast, and low radiation. A summary of normal postoperative chest radiograph findings is summarized in Box 1 and Fig. 1 . Additional imaging strategies include computed tomography (CT), CT angiography, and nuclear medicine (NM) pulmonary perfusion scintigraphy as outlined in Table 1 .
- •
Transverse sternotomy wires
- •
Pulmonary sutures from graft downsizing, particularly in children
- •
Pleural thickening, small pleural effusions, and pneumothoraces are common immediately after transplantation
- •
Nonspecific pulmonary infiltrates are expected in the first 3 days after transplantation
- ○
Primary graft dysfunction affects nearly all patients in the immediate postoperative period
- ○
| Imaging Techniques | Indication | Imaging Pearls |
|---|---|---|
| Chest radiograph |
|
|
| Contrast-enhanced CT |
|
|
| Noncontrast CT |
|
|
| NM pulmonary perfusion scintigraphy and CT angiography |
|
|
Complications
Potential complications of lung transplantation are wide ranging, vary by time period posttransplant, and are of overall similar types in children compared with adults. Early complications (<1 week posttransplant) include primary graft dysfunction, seen in nearly all transplants and manifesting as nonspecific pulmonary opacities. Vascular anastomotic complications, primarily thrombosis, are relatively uncommon and may be evaluated by echocardiography or perfusion scintigraphy. Intermediate complications (1 week to 2 months) include acute rejection, pulmonary thromboembolism, and bronchial anastomotic complications. ,
Bronchial anastomotic complications
The bronchial anastomoses are particularly vulnerable to ischemic complications, as the bronchial arteries are typically not reconnected following transplantation. , There may be a mismatch between the donor and recipient bronchial sizes, which can be addressed surgically by downsizing via sutures, or by overlapping/telescoping anastomotic techniques. , Anastomoses are typically covered with vascularized pericardial or parabronchial tissue to minimize risk of dehiscence. Airway stenosis or malacia may result in poor sputum clearance and repeated infections, requiring recurrent bronchoscopy and stenting.
Bronchial anastomotic dehiscence
Anastomotic dehiscence is a rare complication (1%–10%); however, results in high morbidity and mortality. Bronchoscopy is the gold standard for both clinically suspected and occult dehiscence, as affected patients are often asymptomatic with normal imaging studies. , However, radiographic findings suggestive of dehiscence include delayed presentation of pneumomediastinum or pneumothorax on chest radiograph, as well as bronchial wall irregularities and extraluminal gas on CT. However, it is important to recognize that small linear air pockets may be seen adjacent to normal telescoping bronchial anastomoses.
Bronchial anastomotic stenosis
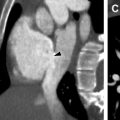
Anastomotic strictures are the most common airway complication, occurring in approximately 10% of patients. , Significant stenosis is defined as focal airway narrowing of greater than 70% without improvement with inspiration or expiration. Bronchial stenosis may be suggested on chest radiograph with recurrent lobar collapse or post obstructive pneumonia. CT is more sensitive, and is useful for preintervention planning by demonstrating the site of focal bronchial narrowing ( Fig. 3 ).

Bronchomalacia
Bronchomalacia is a less common airway complication, occurring in approximately 3% of patients, frequently within the first 4 postoperative months. Bronchomalacia represents dynamic airway narrowing of greater than 50% between inspiration and expiration, which may be best demonstrated on bronchoscopy. Inspiration and expiration CT or dynamic CT acquisitions also may demonstrate bronchomalacia in a cooperative patient. Indirect findings on chest radiograph include areas of segmental atelectasis or recurrent infiltrate.
Chronic lung allograft dysfunction
Chronic lung allograft dysfunction (CLAD) remains a significant challenge in all patients with lung transplantation, but is accentuated in children due to their generally longer life expectancy. Long-term survival of pediatric patients with lung transplantation remains lower than that of other pediatric solid organ transplants, with 5-year survival for lung transplant approximately 60% compared with 85% in liver and 98% in kidney transplants. , , CLAD is the main reason for this disparity, and eventually affects most patients with lung transplantation. , No well-proven therapy exists for CLAD. Although bronchiolitis obliterans may be the most familiar form of CLAD, the term encompasses multiple variants, including obstructive and restrictive patterns. The presence of CLAD is defined clinically by spirometry values. Imaging studies demonstrate poor sensitivity for initial disease detection; however, CT evaluation may help differentiate between forms of CLAD, as well as provide additional data as to disease progression.
Bronchiolitis obliterans syndrome
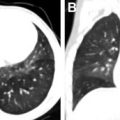
Bronchiolitis obliterans syndrome (BOS) is the most common form of CLAD, and is characterized by progressive airflow obstruction with decreased forced expiratory volume on spirometry. Chest radiographs are often normal. High-resolution CT (HRCT) demonstrates bronchiectasis, bronchial wall thickening, and mosaic air trapping on expiratory sequences ( Fig. 4 ).

Azithromycin-reversible allograft dysfunction
Azithromycin-reversible allograft dysfunction (ARAD) is a reversible form of allograft dysfunction characterized by improvement in spirometry and radiographic abnormalities following treatment with azithromycin. Although the acute features are reversible, ARAD has been shown to correlate with impaired long-term survival. HRCT findings of ARAD overlap with BOS, and include geographic air trapping, bronchial wall thickening, and bronchiectasis. However, the presence centrilobular and tree in bud micronodules may be helpful in differentiating ARAD from BOS, although acute infection must be excluded. Micronodules that are seen with ARAD on CT should resolve following azithromycin treatment.
Restrictive allograft syndrome
Restrictive allograft syndrome (RAS) is a restrictive/fibrotic form of CLAD that affects approximately 30% of transplant recipients. Compared with BOS, patients with RAS have a poorer prognosis, with a median survival of less than 2 years. RAS demonstrates an upper lobe predilection, with a fibrotic pattern on imaging. Chest radiograph may reveal persistent upper lobe–predominant infiltrates with signs of volume loss. HRCT may demonstrate upper lobe–predominant peripheral consolidations, septal and subpleural thickening, reticular-nodular or mosaic ground-glass opacities, peripheral predominant traction bronchiectasis, and air trapping. ,
Acute fibrinoid organizing pneumonia
Acute fibrinoid organizing pneumonia (AFOP) is an additional rare form of restrictive lung injury that occurs more frequently in patients with lung transplantation. HRCT demonstrates consolidations and ground-glass opacities with interlobular septal thickening. Given its rarity compared with infection, AFOP should be considered in the presence of persistent radiographic findings with absent infectious symptoms or lack of response to infectious therapies.
Infections
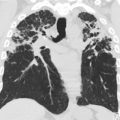
Infection is a significant challenge following lung transplantation. Multiple factors contribute to the high rate of posttransplant infection, including high level of immunosuppression, graft exposure to infectious agents via the airway, and reduced mucociliary and lymphatic clearance. In addition, patients with cystic fibrosis may be colonized with atypical or resistant organisms ( Fig. 5 ). A full description of the varied imaging findings seen in pulmonary infections is beyond the scope of this article. However, radiologists should be familiar with the common trends in infection etiology by time of onset posttransplantation.

Within the first month posttransplantation, bacterial and fungal infections are commonly seen. Angioinvasive aspergillus is a particularly morbid fungal infection that can occur during this period. Viral pneumonias commonly occur in the second to sixth months posttransplantation. Viral respiratory infections are particularly common following pediatric lung transplantation, and are associated with decreased 1-year survival. Immunosuppression is typically lower following the sixth month, leading to fewer opportunistic infections. Late infections frequently include community-acquired viral or bacterial organisms, as well as reactivation of latent Mycobacterium tuberculosis .
Liver transplantation
Orthotopic liver transplantation (OLT) is the treatment of choice for children and adults with end-stage liver disease. In the adult population, the etiology of liver failure is frequently viral hepatitis, nonalcoholic steatosis, or alcoholic liver disease. In contrast, in children, cholestatic liver disease, particularly biliary atresia, is the most common cause of liver failure. Other indications for OLT in children include acute liver failure, primary sclerosing cholangitis, and malignancy such as hepatoblastoma. Pediatric OLT remains a complicated surgery with many potential complications; however, 5-year survival remains greater than 80%. In contrast, overall adult OLT 5-year survival is close to 75%. Intermediate and late-term complications include infection, biliary tract obstruction, vascular compromise, rejection, lymphoproliferative disease, and recurrence of the original disease.
Surgical Techniques
Surgical technique can vary widely, particularly in pediatric OLT, depending on patient and donor size as well as vascular and biliary anatomy. A full understanding of each patient’s postoperative anatomy is essential for imaging evaluation. In addition to the typical whole-liver transplant performed in adult patients, children may receive split liver or segmental grafts. Left lateral segment transplants may be positioned with the neo-porta hepatis at the right lateral aspect of the graft within the far right lateral peritoneal cavity, leading to a characteristic position of the biliary and vascular structures within the abdomen ( Fig. 6 ).

Although hepatic arterial (HA) anastomoses are typically performed end-to-end in a fishmouth fashion, vascular conduits arising from the infrarenal aorta may be used if the recipient’s hepatic artery is too small, or in the case of recurrent transplant or HA thrombosis. In the presence of accessory hepatic arteries, multiple anastomoses may be performed. An end-to-end anastomosis is typically performed between the main portal vein (PV) of the recipient and donor. However, interposition grafts may be used in the setting of PV thrombosis or when there is substantial caliber discrepancy between the recipient and donor PV. Hepatic vein (HV) anastomosis is frequently performed in a “piggyback” technique in children, with end-to-side or side-to-side anastomosis of the donor HVs or inferior vena cava (IVC) to the preserved recipient IVC ( Fig. 7 ).

Because of the high prevalence of biliary atresia in the pediatric OLT population, biliary reconstruction is frequently performed via hepaticojejunostomy in a Roux-en-Y configuration. End-to-end common bile duct anastomoses may be performed in the absence of underlying biliary disease.
Imaging Techniques and Normal Findings
Sonography is rapid and easily accessible, and is therefore the first choice for the evaluation of liver transplants in the immediate and late postoperative periods ( Fig. 8 ). A summary of normal sonographic findings following liver transplantation is listed in Box 2 . Additional imaging strategies for the evaluation of complications include the use of CT, MR, and NM, as outlined in Table 2 .