CHAPTER 8 Imaging Evaluation of Acute Abdominal Pain
ACUTE ABDOMINAL PAIN: A DIAGNOSTIC DILEMMA
Acute abdominal pain is a common clinical syndrome and a diagnostic dilemma for clinicians. For centuries, physicians relied on obtaining a history of illness that elicited characteristic clinical symptoms. This history, combined with careful elicitation of physical examination findings, allowed physicians to discriminate among many potential disease processes. Unfortunately, historical, physical examination, and laboratory data are often nonspecific and inconclusive, spurring a shift in the diagnostic approach to patients with abdominal pain toward imaging. As with physical examination, image interpretation is facilitated by familiarity with the relevant anatomic structures and common diagnoses to be considered in patients presenting with specific pain syndromes (Table 8-1).
Table 8-1 Causes of Acute Abdominal Pain Based on Location of Symptoms
| Location of Pain | Relevant Structures and Common Diagnoses to Consider |
|---|---|
| Right upper quadrant | Bile ducts: obstruction, choledocholithiasis, cholangitis, bile leak, hemobilia from biopsy or other trauma |
| Left upper quadrant | |
| Midepigastric | |
| Right lower quadrant | Colon: colitis, diverticulitis, epiploic appendagitis, perforated tumor, perforated foreign body, ischemia |
| Left lower quadrant | |
| Generalized | Bowel: obstruction (neoplastic, inflammatory, or volvulus), ileus, ischemia, perforation, inflammation |
| Flank and back | Kidney and ureters: ureterolithiasis, pyelonephritis/abscess, embolism/infarction, venous thrombosis, cyst rupture, hemorrhage, nephritis |
| Pelvis and groin | |
| Scrotal or inguinal |
Keep in mind that significant overlap exists between the diseases listed in Table 8-1 and their clinical presentations. A confusing influence in the diagnosis of abdominal pain is related to the overlapping innervation of the abdominal organs. For example, there exists considerable overlapping innervation of the stomach (T5-7), biliary tract (T6-8), and pancreas (T5-9). Therefore, a gastric process can be difficult to differentiate from biliary colic or pancreatitis based on location of pain alone. Overlapping innervation of structures such as the colon (T10-L1), small bowel (T8-10), and kidney (T10-L1) can obscure the precise origin of pain elsewhere in the abdomen as well. Also, remember that abdominal organs do not always reside in their expected locations. For example, the cecum and appendix have a notorious propensity to extend to locations beyond the right lower quadrant, and patients with situs anomalies or bowel malrotation are not uncommon. Finally, disease processes often present remote from their organ of origin. Inflammation and tumor can spread via the subperitoneal space within the ligaments and mesenteries of the abdomen and pelvis or via the peritoneal space. Knowledge of these pathways of disease spread as discussed in Chapter 6 can greatly facilitate image interpretation and explain apparent discrepancies between the clinical presentation and the actual diagnosis.
Not all imaging modalities are equally capable of demonstrating the structures and disease processes listed in Table 8-1. For example, computed tomography (CT) would be an inappropriate first test to perform in a patient presenting with scrotal pain, whereas transabdominal ultrasound would be a poor choice for excluding peptic ulcer disease. Therefore, when imaging is deemed necessary for further evaluation of a patient’s pain, the first decision to make involves the choice of imaging modality. A carefully optimized protocol must be selected and combined with appropriate patient preparation to maximize the effectiveness of the chosen modality.
COMPUTED TOMOGRAPHY: THE WORKHORSE OF THE EMERGENCY DEPARTMENT
Why Computed Tomography Rules the Emergency Department
Despite the wide variety of available imaging options, CT has been adopted as the modality of choice for the evaluation of patients presenting with acute abdominal pain not clearly localized to the right upper quadrant or pelvis (in females of child-bearing age). The use of plain radiographs in the acute setting has come under criticism, mainly because of low sensitivity of conventional radiography for most common causes of abdominal pain. Some studies recommend use of unenhanced CT of the abdomen and pelvis rather than abdominal radiography, arguing that plain films rarely provide a specific diagnosis. CT is much more likely than plain radiographs to show a specific cause of abdominal pain and can be useful in distinguishing between those processes that require urgent surgical intervention and those suitable for conservative management. Several studies have shown that routine evaluation of the abdomen and pelvis with CT in the emergency setting decreases both the number of hospital admissions and the length of stay in the emergency department.
Computed Tomography Protocol Considerations for Acute Abdominal Pain
Traditional positive oral contrast (high attenuation) is useful for distinguishing bowel from other structures and for demonstrating perforation of the gastrointestinal tract. Negative oral contrast (low attenuation) accompanied by injection of intravenous contrast media can be useful for demonstrating inflammation and other enhancing lesions of the bowel wall (Fig. 8-1). When urgency dictates, examinations can be performed without enteric contrast media.
Helpful Computed Tomographic Findings in the Acute Abdomen
Because of the all-inclusive survey provided by CT and the overlapping clinical presentations that can cloud the origins of abdominal pain, radiologists must be facile with interpreting CT scans of the abdomen and pelvis. This skill requires a systematic approach and knowledge of findings that aid in disease localization. A number of signs have been proved effective in localizing the source of acute abdominal pain (Box 8-1).
Focal Fat Stranding or Fascial Thickening
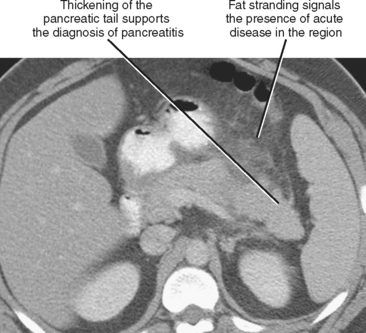
Fat stranding adjacent to a bowel loop or solid organ is often a key CT finding indicating the origin of pain. Stranding is seen as linear or hazy increased attenuation within a region of fat, usually caused by fluid or inflammatory cells infiltrating the tissues (Fig. 8-2) and often accompanied by thickening of nearby fascial planes. Stranding is most useful when it is localized because generalized stranding is nonspecific and can be seen with any systemic cause of edema (e.g., congestive heart failure or hypoproteinemia). Focal fat stranding can signal a focus of acute disease and should always prompt focused inspection of the adjacent structures.
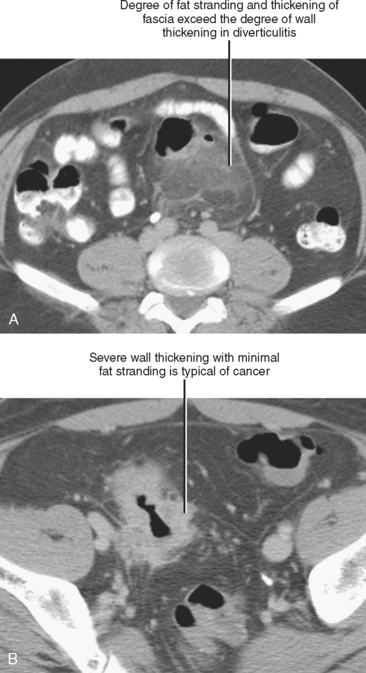
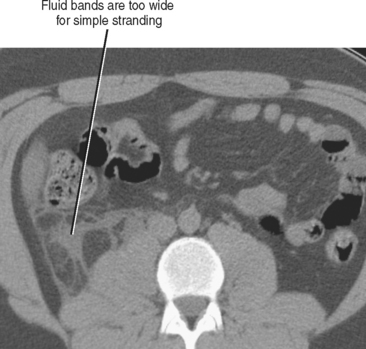
Disproportionate fat stranding refers to focal stranding adjacent to bowel associated with minimal or absent bowel wall thickening. Its presence suggests entities that typically result in a greater degree of inflammation in the adjacent mesentery than in the bowel wall itself. The presence or absence of disproportionate fat stranding is one method used to differentiate colonic diverticulitis from colon cancer, because diverticulitis tends to result in a greater degree of pericolic inflammation relative to the degree of bowel wall thickening (Fig. 8-3). Disease processes associated with disproportionate fat stranding are listed in Table 8-2.
Table 8-2 Disease Processes Associated with Disproportionate Fat Stranding
| Diagnosis | Clinical Discriminators | Imaging Discriminators |
|---|---|---|
| Appendicitis | Right lower quadrant pain, nausea, fever, leukocytosis | Periappendiceal inflammation |
| Diverticulitis | Left lower quadrant pain, fever, leukocytosis | Pericolonic inflammation, adjacent diverticulum |
| Epiploic appendagitis | Nausea and fever are rare | Fatty pericolonic mass with dense rim and central high-attenuation focus |
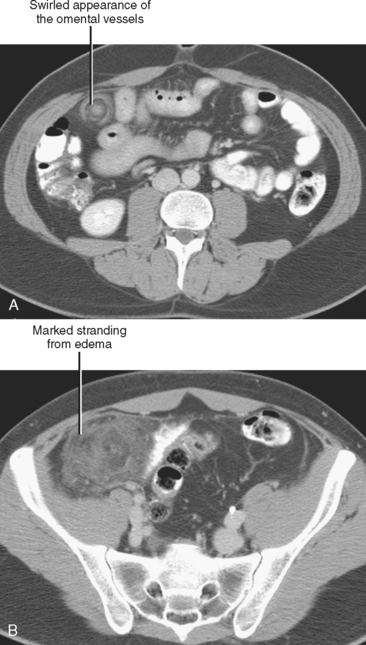
| Omental infarction | Triangular or ovoid fatty/heterogeneous lesion deep to right anterior abdominal wall |
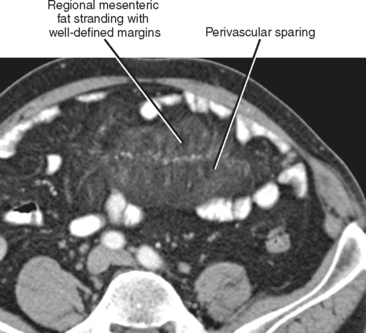
Mesenteric fat stranding can result from a broad variety of inflammatory and neoplastic disease processes; therefore, it is important to consider this finding in the context of clinical presentation and other imaging findings (Fig. 8-4). The most common causes of mesenteric fat stranding are listed in Box 8-2.
Sclerosing mesenteritis is an uncommon primary inflammatory disorder of the mesentery. An elusive disease that can be difficult to diagnose and to treat, sclerosing mesenteritis has been known by many names, including mesenteric panniculitis, retractile mesenteritis, fibrosing mesenteritis, mesenteric lipodystrophy, and mesenteric Weber–Christian disease. The most common CT finding is fat stranding in the mesentery, a nonspecific finding sometimes referred to as a “misty” mesentery (Fig. 8-5). Additional findings of sclerosing mesenteritis may include: (1) a “fat halo,” or ring of low attenuation surrounding mesenteric vessels within a region of mesenteric fat stranding; (2) a dense pseudocapsule surrounding the area of involved fat; and (3) small soft-tissue nodules within the affected region of mesentery.
Segmental Bowel Dilatation
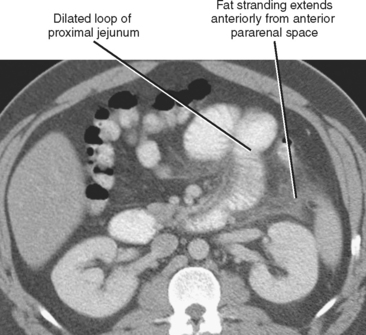
Adynamic or paralytic ileus is often associated with a generalized decrease in bowel motility, resulting in relatively uniform distention of the small intestine and colon. However, a focal area of inflammation in the peritoneal cavity can result in segmentally decreased motility in bowel loops immediately adjacent to the acute process. This accounts for the “sentinel loop” sign associated with intraabdominal inflammation (Fig. 8-6) and can be a clue to the nature and location of the underlying disease process.
Segmental Bowel Wall Thickening
Distinguishing between normal and abnormal bowel wall thickness is one of the greatest challenges in abdominal imaging. Even the combination of a diligent technologist and compliant patient rarely results in uniform bowel distention throughout the gastrointestinal tract. Because some bowel loops are likely to be incompletely distended during the examination, it is common to mistakenly describe a collapsed bowel segment as “thickened.” Of course, if one becomes accustomed to ignoring apparently thickened loops of bowel, critical diagnoses can be missed.
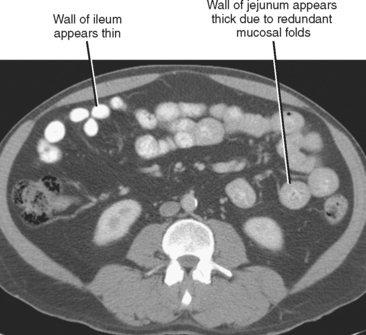
Be cautious about calling wall thickening in the jejunum on CT examinations. The normal jejunum contains many more redundant folds than ileum. The CT correlate of redundant folds in the jejunum is a thicker appearance of the bowel wall (Fig. 8-7). As stated earlier, looking for areas of nondependent air between folds may help distinguish normal from thickened jejunum.
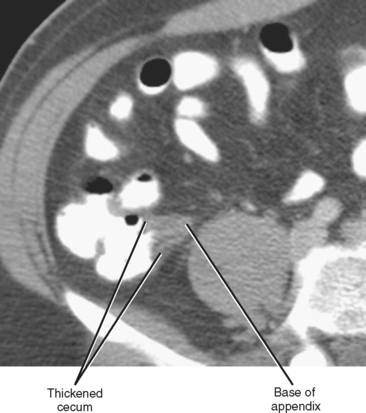
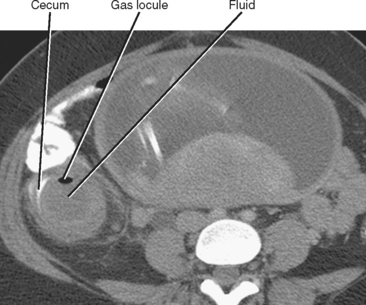
Whereas thickening of the entire cecum can be seen with colitis or colon cancer, focal thickening of the cecal apex near the base of the appendix is often seen with acute appendicitis. Sometimes called the cecal arrow sign, wall thickening at the junction of the cecum and appendix causes the lumen of the cecum to point toward the appendix, supporting the diagnosis of acute appendicitis (Fig. 8-8).
Table 8-3 lists the disease processes most commonly associated with segmental thickening of the bowel wall on CT, as well as some parameters that can be used to refine the differential diagnosis.
Table 8-3 Diseases Associated with Segmental Bowel Wall Thickening
| Diagnosis | Imaging Discriminators |
|---|---|
| Appendicitis | |
| Crohn disease | |
| Colonic diverticulitis | |
| Gastritis | |
| Malignancy | |
| Meckel’s diverticulitis | Blind ending loop from ileum, not from cecum |
Extraluminal Gas
PNEUMOPERITONEUM
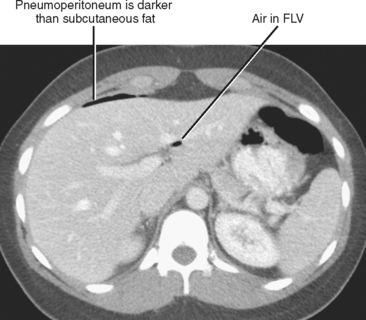
The interpretation of CT examinations performed for acute abdominal pain should always include a directed search for peritoneal gas. Gas usually rises to the most nondependent recesses of the peritoneal cavity; therefore, a focused survey along the anterior peritoneal surface can increase sensitivity. During the search, it is helpful to use window and level settings that allow differentiation between fat and gas. The peritoneal space extends into the fissures for the ligamentum teres and ligamentum venosum, so include those locations in the search pattern. When gas is present in these fissures, duodenal or distal stomach perforation is often the source (Fig. 8-9). The most common causes of pneumoperitoneum are listed in Table 8-4.
Table 8-4 Causes of Pneumoperitoneum
| Surgical Causes (90%)* | Nonsurgical Causes (10%) |
|---|---|
* Requirement for urgent, often surgical, therapy.
PNEUMORETROPERITONEUM
Extraperitoneal gas is usually the result of bowel perforation or infection. Because gas is more restricted within the extraperitoneal compartments than within the peritoneal cavity, the location of gas within the retroperitoneum is often more useful for identifying the source. In general, by determining whether the gas is within the anterior pararenal space, the perirenal space, or posterior pararenal space, and noting whether it is unilateral or bilateral, the differential diagnosis can be narrowed considerably. The most likely causes for retroperitoneal gas within each space are outlined in Table 8-5.
Table 8-5 Correlation between Location of Extraperitoneal Gas and Its Source
| Compartment | Side | Likely Source |
|---|---|---|
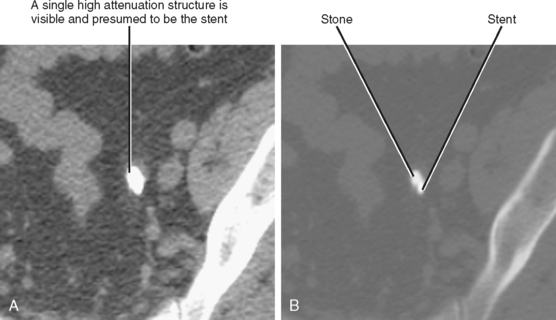
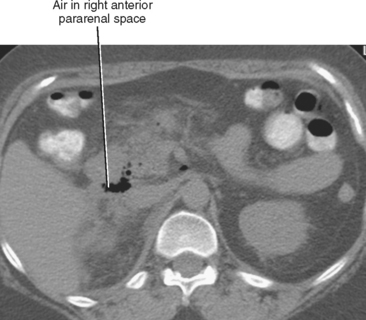
| Anterior pararenal | Right | Perforation of descending duodenum (e.g., duodenal ulcer or recent ERCP) (Fig. 8-10) |
| Left | Perforation of descending or sigmoid colon (e.g., diverticulitis) | |
| Bilateral | ||
| Perirenal | Right or left | Renal infection |
| Posterior pararenal | Left | Perforated sigmoid diverticulitis |
| Bilateral |
INTRAMURAL GAS COLLECTIONS
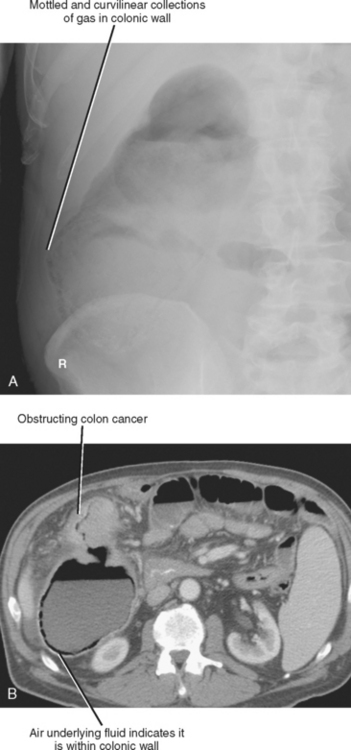
Pneumatosis intestinalis describes gas in the wall of the alimentary tract. Because the gas is confined between the mucosal and serosal layers, pneumatosis does not move freely through the abdomen and is usually confined to one region of the intestinal tract. On plain film, pneumatosis appears as a combination of curvilinear and mottled gas, and can easily be confused with fecal material (Fig. 8-12). Although the curvilinear shape of the gas collections are better appreciated by CT, differentiation from fecal material can still be challenging. Because the mucosal and serosal layers can be thin when distended, pneumatosis can be difficult to detect when located adjacent to gas within the bowel lumen. However, when fluid is present adjacent to intramural gas, the gas is much more conspicuous.
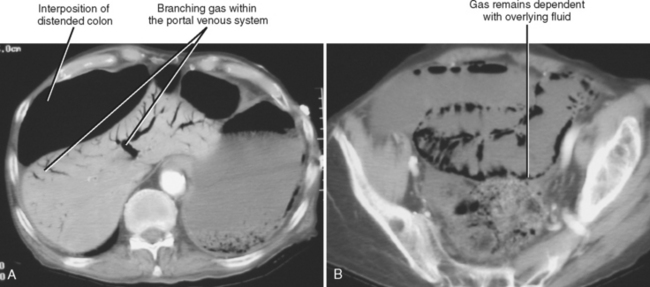
Most of the many causes of intestinal pneumatosis are listed in Table 8-6. Pneumatosis is often seen in association with acute gastrointestinal disease processes such as bowel ischemia, bowel obstruction, and severe bowel infections. When seen in the presence of bowel ischemia, it is associated with reported mortality rates of 50% to 75%. However, pneumatosis is also associated with chronic disease processes such as obstructive pulmonary disease, Whipple disease, and scleroderma. Iatrogenic pneumatosis can result from endoscopy with or without biopsy, barium enema, or bowel surgery, particularly if a bowel anastomosis is performed. Occasionally, idiopathic pneumatosis is seen as an unsuspected finding in patients without acute abdominal symptoms. When pneumatosis is present, always look at the mesenteric vessels and liver for evidence of portal venous gas (Fig. 8-13).
Table 8-6 Causes of Intestinal Pneumatosis
| Causes that Usually | Causes that Usually |
|---|---|
| Require Urgent Therapy | Do Not Require Therapy |
OTHER GAS COLLECTIONS
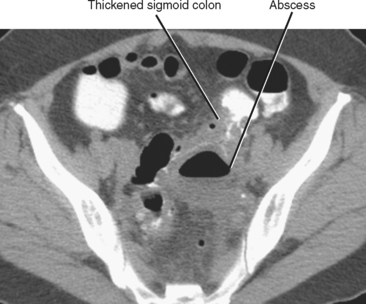
A contained collection of extraluminal gas near bowel is a common sign of perforation with or without abscess formation. Abscesses contain infected material with variable proportions of fluid, debris, and gas (Fig. 8-14). Common causes of abscess include bowel perforation, superinfected postoperative fluid, hematogenous dissemination of bacteria, pyelonephritis, or anastomotic leak. Because the bowel also contains differing proportions of gas, fluid, and semisolid material, it is sometimes mistaken for an abscess. A key feature that distinguishes an abscess from bowel is discontinuity or eccentric location with respect to the alimentary tract. When an abscess is identified near a bowel loop without evidence of diverticula or inflammatory bowel disease, be certain to look for a foreign body such as a fish bone.
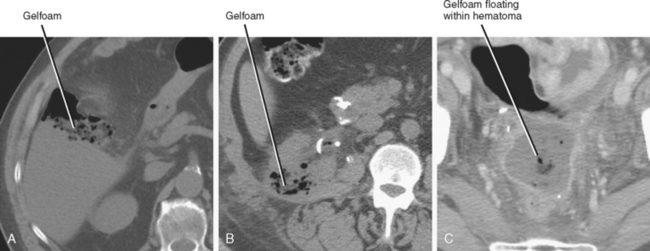
Gelatin bioabsorbable sponge (Gelfoam) and oxidized cellulose (Surgicel) used for intraoperative hemostasis can result in an appearance similar to abscess on CT. Clues to the presence of these materials on CT include a history of recent surgery, linear organization of small gas bubbles (best seen on bone windows), stable appearance of the gas on sequential examinations, and unusual shape of the collection. Because most postoperative abscesses contain at least some fluid, a predominantly mottled gas appearance and the absence of an air–fluid level favor hemostatic material (Fig. 8-15). This general rule does not work in reverse, however; because the material may be surrounded by hematoma, the presence of fluid does not necessarily indicate abscess. Note that this material can occasionally become superinfected, but because it is semisolid and typically in an area of recent hemorrhage, catheter drainage is not usually performed.
Focal Fluid Collections
Fluid collecting adjacent to thickened or inflamed bowel often indicates bowel perforation with or without abscess formation. For example, although acute appendicitis and diverticulitis without perforation can result in inflammation of the adjacent fat, a nonlinear fluid collection near a thickened appendix or inflamed diverticulum increases the likelihood of perforation (Fig. 8-16).
A similar principle can be applied to perirenal collections. Urinary obstruction often results in stranding of the perinephric fat, appearing as thin linear and curvilinear areas of fluid attenuation within otherwise low-density fat. If the areas of fluid attenuation become wider or rounded in the setting of urinary obstruction, forniceal rupture should be suspected (Fig. 8-17).
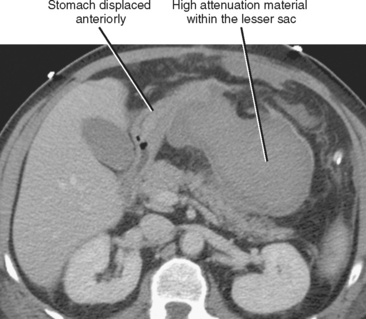
The presence of fluid in the lesser sac presents a limited differential diagnosis, particularly if there is little or no fluid in the rest of the peritoneal cavity. The most likely causes of fluid in the lesser sac include processes that affect the pancreas, stomach or gastroesophageal junction (Fig. 8-18), duodenum, or spleen.
As mentioned earlier, the pelvis is the most common site for physiologic fluid to accumulate. In addition, peritoneal fluid from inflammatory or malignant disease processes originating anywhere within the peritoneal space often accumulates first within the pelvis because of its dependent location. Primary pelvic processes that result in focal pelvic fluid collections include pelvic inflammatory disease (PID), endometriosis, ovarian cancer, bladder injury, and pelvic surgery.
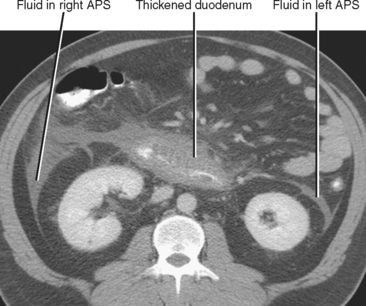
The anterior pararenal space contains the pancreas, the ascending and descending colon, and the extraperitoneal portion of the duodenum. Therefore, fluid in the anterior pararenal space is likely to originate from these structures. Although the anterior pararenal space is continuous across the midline, fluid collections from the duodenum and colon are often confined to the side of origin. Bilateral anterior pararenal fluid collections usually are of pancreatic origin (Fig. 8-19).
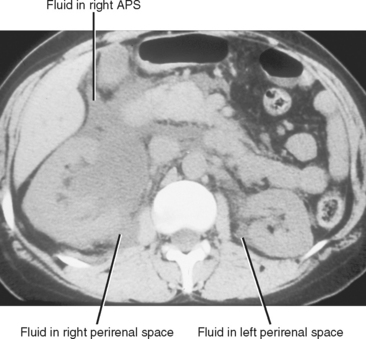
The perirenal space contains the kidneys and adrenal glands, and is divided into right and left because the anterior renal fascia fuses with the fascia surrounding the abdominal aorta and inferior vena cava. Despite this compartmentalization, fluid under pressure within the perirenal space can occasionally cross to the contralateral side (Fig. 8-20). The perirenal space extends caudally to the upper pelvis, where it communicates with the anterior pararenal space. Despite these potential avenues of communication, the majority of perirenal fluid collections remain confined to the perirenal space.
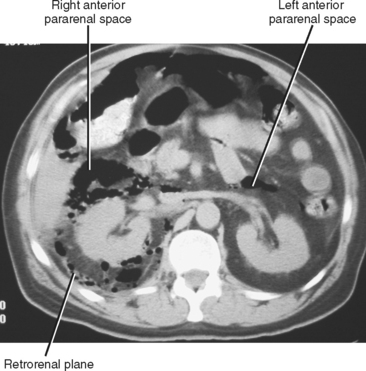
An additional potential space exists between the two layers of the posterior renal fascia, sometimes called the retrorenal plane. Fluid collections in this potential space most often arise from pancreatitis (Fig. 8-21) and are in continuity with the anterior pararenal space (see Fig. 6-33).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree